What Type Of Ions Do Bases Release
Muz Play
Mar 20, 2025 · 5 min read
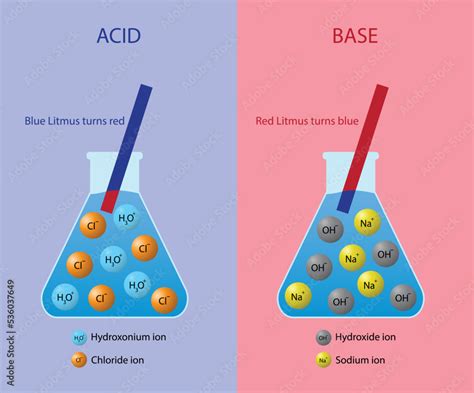
Table of Contents
What Type of Ions Do Bases Release? Understanding Arrhenius, Brønsted-Lowry, and Lewis Bases
Understanding the fundamental nature of bases is crucial in chemistry. A common question that arises is: what type of ions do bases release? The answer isn't as straightforward as it might seem, as the definition of a base depends on the theoretical framework being used. This article explores the different definitions of bases – Arrhenius, Brønsted-Lowry, and Lewis – and clarifies the types of ions, if any, they release.
Arrhenius Bases: The Hydroxide Ion Connection
The simplest definition of a base comes from the Arrhenius theory. According to Arrhenius, a base is a substance that dissociates in water to produce hydroxide ions (OH⁻). This definition is historically significant and provides a clear, easily understandable picture for many common bases.
Examples of Arrhenius Bases and their Dissociation:
- Sodium hydroxide (NaOH): A strong Arrhenius base that completely dissociates in water: NaOH(aq) → Na⁺(aq) + OH⁻(aq). Notice the release of the hydroxide ion.
- Potassium hydroxide (KOH): Another strong Arrhenius base, undergoing complete dissociation: KOH(aq) → K⁺(aq) + OH⁻(aq), again releasing the hydroxide ion.
- Calcium hydroxide (Ca(OH)₂): This is a weaker Arrhenius base, meaning it doesn't dissociate completely. However, the dissociation produces hydroxide ions: Ca(OH)₂(aq) ⇌ Ca²⁺(aq) + 2OH⁻(aq). The equilibrium indicates that not all Ca(OH)₂ molecules break apart.
Key Takeaway: The defining characteristic of Arrhenius bases is their production of hydroxide ions (OH⁻) upon dissolution in water. Other ions may also be present (like Na⁺ or K⁺ in the examples above), but the OH⁻ ion is essential for its classification as an Arrhenius base.
Limitations of the Arrhenius Definition
While the Arrhenius definition is useful for understanding basic chemistry, it has limitations:
- Water dependency: The definition is strictly limited to aqueous solutions. Many substances act as bases in non-aqueous solvents but wouldn't be classified as Arrhenius bases because they don't produce OH⁻ ions in water.
- Narrow scope: The definition excludes many compounds that exhibit basic properties but don't contain hydroxide ions.
These limitations led to the development of more comprehensive definitions of bases, such as the Brønsted-Lowry theory.
Brønsted-Lowry Bases: Proton Acceptors
The Brønsted-Lowry theory provides a broader definition of acids and bases. A Brønsted-Lowry base is defined as a proton (H⁺) acceptor. This definition overcomes the limitations of the Arrhenius theory by not requiring the presence of hydroxide ions or an aqueous solution.
Understanding Proton Acceptance:
A Brønsted-Lowry base accepts a proton from an acid, forming a conjugate acid. This reaction doesn't necessarily release an ion in the same way an Arrhenius base does. Instead, it involves a change in the base's structure as it accepts the proton.
Examples of Brønsted-Lowry Bases and their Reactions:
- Ammonia (NH₃): Ammonia acts as a Brønsted-Lowry base when it accepts a proton from water: NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq). Note that while OH⁻ is produced, it's a result of the proton transfer, not a direct release from ammonia itself. Ammonia accepts a proton, becoming NH₄⁺ (its conjugate acid).
- Bicarbonate ion (HCO₃⁻): This ion can act as a Brønsted-Lowry base by accepting a proton: HCO₃⁻(aq) + H⁺(aq) → H₂CO₃(aq). Again, no specific ion is released; instead, a proton is accepted.
- Many organic molecules: Many organic molecules containing nitrogen or oxygen atoms with lone pairs of electrons can act as Brønsted-Lowry bases by accepting protons.
Key Takeaway: Brønsted-Lowry bases don't necessarily release specific ions. Their defining characteristic is their ability to accept protons, leading to a change in their chemical structure and often the formation of a conjugate acid.
Lewis Bases: Electron Pair Donors
The Lewis theory offers the broadest definition of acids and bases. A Lewis base is defined as an electron-pair donor. This definition encompasses a vast range of substances, including those not covered by the Arrhenius or Brønsted-Lowry definitions. Lewis bases don't necessarily release ions; their defining feature is the donation of an electron pair.
Understanding Electron Pair Donation:
Lewis bases have at least one lone pair of electrons that they can donate to a Lewis acid (an electron-pair acceptor). The interaction forms a coordinate covalent bond.
Examples of Lewis Bases and their Reactions:
- Ammonia (NH₃): Ammonia acts as a Lewis base because of its lone pair of electrons on the nitrogen atom. It can donate this pair to a Lewis acid.
- Water (H₂O): Water has two lone pairs of electrons on the oxygen atom, making it a Lewis base.
- Many anions: Many negatively charged ions (anions) can act as Lewis bases because they have excess electrons.
- Many organic molecules: Similar to Brønsted-Lowry bases, many organic molecules with lone pairs on oxygen, nitrogen, or other atoms, can act as Lewis bases.
Key Takeaway: Lewis bases don't release ions in the traditional sense. Their defining feature is their ability to donate a lone pair of electrons to form a coordinate covalent bond with a Lewis acid. This broader definition includes many substances that wouldn't be considered bases under the Arrhenius or Brønsted-Lowry theories.
Comparing the Definitions: A Summary Table
| Definition | Defining Characteristic | Ion Release? | Examples |
|---|---|---|---|
| Arrhenius | Produces OH⁻ in water | Yes (OH⁻) | NaOH, KOH, Ca(OH)₂ |
| Brønsted-Lowry | Accepts a proton (H⁺) | Not necessarily | NH₃, HCO₃⁻, many organic molecules |
| Lewis | Donates an electron pair | Not necessarily | NH₃, H₂O, many anions, many organic molecules |
Conclusion: A Spectrum of Basicity
The type of ions released by a base depends heavily on the definition used. Arrhenius bases specifically release hydroxide ions (OH⁻) in water. Brønsted-Lowry and Lewis bases don't necessarily release ions; instead, they engage in proton acceptance or electron pair donation, respectively. The Lewis definition is the most comprehensive, encompassing a vast range of compounds exhibiting basic properties. Understanding these different definitions allows for a complete grasp of the diverse world of bases in chemistry. The key takeaway is to appreciate that basicity isn't solely about ion release; it's about the ability to interact with acids through proton acceptance or electron pair donation. This understanding is fundamental to comprehending numerous chemical reactions and processes.
Latest Posts
Latest Posts
-
Measurement Of The Amount Of Matter In An Object
Mar 20, 2025
-
Significant Figures Addition And Subtraction Practice
Mar 20, 2025
-
How To Calculate Ph At The Equivalence Point
Mar 20, 2025
-
Conversion From Rectangular To Spherical Coordinates
Mar 20, 2025
-
How Does A Magnifying Glass Work Physics
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Ions Do Bases Release . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
