How To Calculate Ph At The Equivalence Point
Muz Play
Mar 20, 2025 · 6 min read
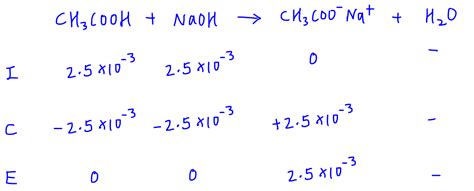
Table of Contents
How to Calculate pH at the Equivalence Point of a Titration
Determining the pH at the equivalence point of a titration is crucial for understanding the reaction's stoichiometry and selecting appropriate indicators. The equivalence point signifies the point where the moles of titrant added exactly equal the moles of analyte present. However, calculating the pH at this point isn't always straightforward and depends heavily on the nature of the acid and base involved. This comprehensive guide will delve into various scenarios, providing you with the tools and knowledge to accurately calculate the pH at the equivalence point for different titrations.
Understanding the Equivalence Point
Before diving into the calculations, it's essential to understand what the equivalence point represents. In a titration, a solution of known concentration (the titrant) is gradually added to a solution of unknown concentration (the analyte). The equivalence point is reached when the moles of titrant added are stoichiometrically equivalent to the moles of analyte. This doesn't necessarily mean the pH is 7; it depends entirely on the strength of the acid and base involved.
Strong Acid-Strong Base Titration
The simplest case involves the titration of a strong acid with a strong base (or vice versa). Here, the reaction goes to completion, producing water and a neutral salt. At the equivalence point, the solution contains only the salt, and the pH is approximately 7.
Calculating pH:
Since the salt formed from a strong acid and strong base doesn't hydrolyze (doesn't react with water to produce H⁺ or OH⁻ ions), the pH at the equivalence point is determined by the autoionization of water:
- Kw = [H⁺][OH⁻] = 1 x 10⁻¹⁴
At 25°C, [H⁺] = [OH⁻], so:
- [H⁺] = √Kw = 1 x 10⁻⁷ M
Therefore, the pH is:
- pH = -log[H⁺] = 7
This is an idealized scenario; slight deviations from 7 can occur due to ionic strength effects and temperature variations.
Weak Acid-Strong Base Titration
Titrating a weak acid with a strong base is more complex. At the equivalence point, the weak acid is completely neutralized, forming the conjugate base of the weak acid. This conjugate base will react with water, undergoing hydrolysis and producing hydroxide ions (OH⁻), increasing the pH above 7.
Calculating pH:
-
Determine the concentration of the conjugate base: This is done using the stoichiometry of the reaction. The moles of conjugate base formed are equal to the initial moles of weak acid. Divide this by the total volume of the solution (initial volume of weak acid + volume of strong base added at the equivalence point) to obtain the concentration.
-
Calculate the Kb of the conjugate base: The Kb (base dissociation constant) is related to the Ka (acid dissociation constant) of the weak acid by the following equation:
- Kw = Ka x Kb
-
Use an ICE table (Initial, Change, Equilibrium) to determine the hydroxide ion concentration: The ICE table helps in solving the equilibrium expression for the hydrolysis reaction of the conjugate base.
-
Calculate pOH and pH: Use the following equations:
- pOH = -log[OH⁻]
- pH = 14 - pOH
Example: Consider the titration of 0.1 M acetic acid (CH₃COOH, Ka = 1.8 x 10⁻⁵) with 0.1 M NaOH. At the equivalence point, all acetic acid is converted to acetate ions (CH₃COO⁻). The concentration of acetate ions needs to be calculated considering the dilution. Then, using the Kb for acetate, and an ICE table, the [OH⁻] can be calculated. From [OH⁻], pOH and then pH is determined.
Weak Base-Strong Acid Titration
This scenario is analogous to the weak acid-strong base titration. At the equivalence point, the weak base is completely neutralized, forming the conjugate acid, which will hydrolyze, producing hydronium ions (H₃O⁺), resulting in a pH below 7.
Calculating pH:
The approach is very similar to the weak acid-strong base titration, but now you'll use the Ka of the conjugate acid (related to the Kb of the weak base through Kw) and an ICE table to determine the [H₃O⁺] concentration. Then calculate the pH using:
- pH = -log[H₃O⁺]
Polyprotic Acid-Strong Base Titration
Polyprotic acids have multiple ionizable protons. Their titrations have multiple equivalence points, each corresponding to the neutralization of one proton. Calculating the pH at each equivalence point requires considering the different equilibrium reactions and the concentrations of the various species present.
Calculating pH:
The calculation at each equivalence point becomes increasingly complex as the number of protons increases. For a diprotic acid, for instance, the first equivalence point would involve the calculation similar to a weak acid-strong base titration. At the second equivalence point, the calculation would consider the second dissociation constant and the concentrations of the relevant species involved in the reaction of the conjugate base formed at the first equivalence point.
Influence of Ionic Strength
Ionic strength significantly influences the activity coefficients of ions in solution, altering the effective concentrations and consequently affecting the pH. At high ionic strengths, the Debye-Hückel equation or its extensions (like the Davies equation) can be used to correct the concentrations for activity effects, leading to more accurate pH calculations. However, for many introductory calculations, this effect can often be neglected.
Practical Considerations and Approximations
In many cases, simplifications and approximations can be made to facilitate the calculations. For instance:
- Neglecting the autoionization of water: This is valid when the concentration of the conjugate acid or base is significantly higher than 10⁻⁷ M.
- Assuming complete dissociation of strong acids and bases: This is a reasonable approximation in most cases, especially at higher concentrations.
- Using the Henderson-Hasselbalch equation: This approximation is useful for buffer solutions, but only holds true near the half-equivalence point, not exactly at the equivalence point.
Choosing the Right Indicator
The choice of indicator for a titration is crucial to visually identify the equivalence point. Indicators are weak acids or bases that change color over a specific pH range, their "transition range". The indicator's transition range should ideally encompass the pH at the equivalence point. For strong acid-strong base titrations, phenolphthalein is commonly used. However, for weak acid-strong base or weak base-strong acid titrations, the selection requires considering the pH at the equivalence point and choosing an indicator with a transition range that overlaps.
Conclusion
Calculating the pH at the equivalence point of a titration involves understanding the nature of the acid and base involved and applying the relevant equilibrium principles. While strong acid-strong base titrations yield a relatively simple calculation, the pH at the equivalence point of weak acid-strong base or weak base-strong acid titrations requires a more detailed approach using ICE tables, equilibrium constants, and potentially corrections for ionic strength. Accuracy in these calculations is essential not only for theoretical understanding but also for practical applications where precise pH control is critical, such as in analytical chemistry and industrial processes. Remember to always consider the specific context and the potential simplifications or corrections applicable to improve your calculations. With a thorough understanding of the underlying principles, accurate pH calculations at the equivalence point become attainable.
Latest Posts
Latest Posts
-
How To Construct A Frequency Table In Excel
Mar 21, 2025
-
Why Is Hydrogen In Group 1
Mar 21, 2025
-
How Do You Add And Subtract Radicals
Mar 21, 2025
-
What Are The Role Of Operating System
Mar 21, 2025
-
How To Create A Wet Mount Slide
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Ph At The Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
