Why Is Hydrogen In Group 1
Muz Play
Mar 21, 2025 · 5 min read
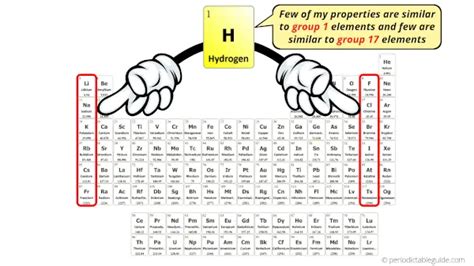
Table of Contents
Why is Hydrogen in Group 1? A Deep Dive into the Periodic Table's Enigma
Hydrogen, the simplest and most abundant element in the universe, occupies a unique and often debated position in the periodic table. While typically placed in Group 1 (alkali metals) due to its single valence electron, its properties significantly differ from the rest of the group members. This article delves deep into the reasons behind hydrogen's placement, explores its anomalous behavior, and examines the alternative arguments for its positioning elsewhere in the periodic table.
The Case for Group 1: The Lone Valence Electron
The primary reason hydrogen finds its place in Group 1 is its electronic configuration. Like alkali metals (lithium, sodium, potassium, etc.), hydrogen possesses a single electron in its outermost shell. This single valence electron is readily lost, forming a +1 cation (H⁺). This similarity in electronic structure is the fundamental basis for its placement in Group 1. The ease with which hydrogen loses its electron explains its reactivity, albeit in a different manner compared to the alkali metals.
Similarities to Alkali Metals:
- Electronic Configuration: The most crucial similarity is the presence of a single electron in the outermost shell (1s¹). This is the defining characteristic of Group 1 elements.
- Cation Formation: Both hydrogen and alkali metals readily lose their valence electron to form a positively charged ion. This ionisation potential, though higher for hydrogen, still demonstrates a similar trend.
- Reactivity: Hydrogen, like alkali metals, readily reacts with non-metals to form compounds. While the reactions might differ in speed and mechanism, the fundamental principle of electron sharing or transfer remains the same.
The Case Against Group 1: Significant Differences
Despite the shared single valence electron, hydrogen displays properties that strikingly contrast with the alkali metals, leading to ongoing debates about its ideal position.
Differences from Alkali Metals:
- Physical State: At standard temperature and pressure, hydrogen exists as a diatomic gas (H₂), while alkali metals are all solid metals. This fundamental difference highlights the significant variations in interatomic forces.
- Metallic Character: Alkali metals are highly reactive, soft, silvery-white metals with excellent electrical and thermal conductivity—characteristics of metallic bonding. Hydrogen, however, lacks these metallic properties; it's a non-metal.
- Ionization Energy: While hydrogen can lose its electron, it possesses a significantly higher ionization energy compared to the alkali metals, making it less willing to lose its electron. This higher energy requirement distinguishes it from the easily ionizable alkali metals.
- Chemical Reactivity: Although both react with non-metals, hydrogen's reactivity differs significantly. While alkali metals vigorously react with water, hydrogen reacts relatively less vigorously, depending on the conditions and the presence of a catalyst. Similarly, hydrogen forms covalent bonds much more readily than the ionic bonds favoured by alkali metals.
- Oxidation States: While hydrogen commonly exhibits a +1 oxidation state like alkali metals, it can also exhibit a -1 oxidation state in compounds like metal hydrides (e.g., NaH). This unique ability to display both positive and negative oxidation states is absent in alkali metals.
Alternative Placement Suggestions:
Given the significant differences, several alternative placement suggestions for hydrogen have been proposed, though none have gained universal acceptance.
Group 17 (Halogens):
Some propose placing hydrogen with the halogens (fluorine, chlorine, bromine, iodine) because it can gain an electron to form a hydride ion (H⁻), similar to how halogens gain an electron to form halide ions. This suggestion is based on the negative oxidation state hydrogen can adopt. However, this placement ignores the vastly different chemical properties hydrogen exhibits compared to the halogens. The electronegativity of hydrogen is much lower than that of the halogens, making this placement unlikely.
A Separate Position:
A more frequently discussed suggestion is to place hydrogen separately altogether, recognizing its unique properties that don't perfectly align with any single group. This approach acknowledges the exceptional nature of hydrogen, highlighting its unique role as a bridging element between metals and non-metals. This placement visually represents its distinct character while emphasizing its unique chemistry.
The Resolution: A Balancing Act
The ongoing debate about hydrogen's placement highlights the limitations of the periodic table's organization. The table is a powerful tool for organizing and understanding the elements, but it's not perfect. Hydrogen's case exemplifies the complexities of classifying elements solely based on a few key properties.
The current practice of placing hydrogen in Group 1, while acknowledging its anomalous behaviour, reflects a compromise. The rationale rests primarily on the shared presence of a single valence electron, which determines many fundamental chemical interactions. However, it's crucial to understand that this placement is not without its limitations and doesn't fully capture the complete chemical diversity of hydrogen. Therefore, the choice represents a trade-off between simplicity and accurate reflection of all chemical properties.
The Importance of Context: Understanding Hydrogen's Uniqueness
Ultimately, the most important aspect of hydrogen's position is to understand that its unique properties defy simple categorization. While Group 1 provides a convenient starting point for understanding its basic reactivity, it’s crucial to recognize its significant differences from the other members of the group.
The key to appreciating hydrogen's chemistry is to understand the context of the reaction. Sometimes, its behavior is consistent with alkali metals, while at other times it behaves entirely differently. This versatility stems from its small size, high ionization energy, and its ability to form both covalent and ionic bonds.
Conclusion: A Continuing Scientific Dialogue
The question of hydrogen's placement remains a valuable point of discussion in chemistry. Its unique properties continue to inspire research and deeper understanding of chemical bonding and reactivity. While the periodic table is a powerful tool, it's essential to recognize its limitations, such as the difficulties in accurately classifying elements like hydrogen. Understanding hydrogen's peculiar position within the table allows us to appreciate the complexity and nuances of the element's chemistry, fueling further scientific exploration and discoveries. The debate surrounding its location serves as a constant reminder of the ever-evolving nature of scientific understanding and the inherent complexity of the natural world. It highlights the importance of considering multiple perspectives and understanding the limitations of any single classification system. Hydrogen, in its unique position, continues to challenge and enrich our understanding of the periodic table and the fundamental building blocks of our universe.
Latest Posts
Latest Posts
-
Is Solid To Liquid Endothermic Or Exothermic
Mar 27, 2025
-
What Does A Negative Enthalpy Mean
Mar 27, 2025
-
Divides The Body Into Anterior And Posterior Portions
Mar 27, 2025
-
Claim Of Fact Value And Policy
Mar 27, 2025
-
How To Calculate Shortage And Surplus
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Why Is Hydrogen In Group 1 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
