Elements In An Element Family Have Similar
Muz Play
Mar 19, 2025 · 6 min read
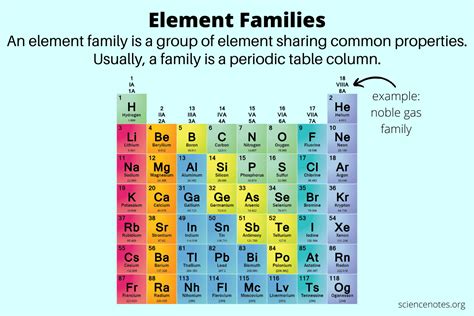
Table of Contents
Elements in an Element Family Have Similar Properties: A Deep Dive into Periodic Trends
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and resulting properties. Elements within the same group, or family, share strikingly similar characteristics. This similarity stems from the commonality in their valence electron configuration, the electrons occupying the outermost shell. Understanding these trends is crucial for predicting the reactivity and behavior of elements, a foundation for numerous applications in chemistry, materials science, and beyond.
Understanding Valence Electrons and Their Role
The valence electrons are the key players in determining an element's chemical behavior. These electrons are loosely bound to the atom and are the first to participate in chemical bonding. Elements in the same family possess the same number of valence electrons, leading to analogous chemical properties. For instance, all alkali metals (Group 1) have one valence electron, making them highly reactive and prone to losing that electron to form a +1 ion.
The Octet Rule and Stability
The drive towards stability significantly influences an element's reactivity. The octet rule, while not universally applicable, states that atoms tend to gain, lose, or share electrons to achieve a full outer electron shell (eight electrons, except for hydrogen and helium which aim for two). Elements in the same family adopt similar strategies to achieve this stable configuration, resulting in similar reactivity patterns.
Exploring Key Periodic Trends
The similarities within element families manifest in several crucial periodic trends:
1. Atomic Radius: Size Matters
The atomic radius, representing the size of an atom, generally increases as you move down a group. This is because each successive element adds another electron shell, increasing the distance between the nucleus and the outermost electrons. Conversely, moving across a period (from left to right), the atomic radius generally decreases. This is due to the increasing nuclear charge pulling the electrons closer to the nucleus, despite adding more electrons to the same shell.
- Example: Cesium (Cs) has a much larger atomic radius than Lithium (Li), both being in Group 1, due to the presence of additional electron shells in Cs.
2. Ionization Energy: The Energy of Removal
Ionization energy is the energy required to remove an electron from a neutral atom. It generally decreases as you move down a group. This is because the outermost electrons are further from the nucleus, experiencing weaker attraction and requiring less energy for removal. Moving across a period, ionization energy generally increases due to the stronger nuclear pull on the electrons.
- Example: It's easier to remove an electron from Cesium than from Lithium because of the increased atomic size and weaker nuclear attraction in Cesium.
3. Electronegativity: Electron-Hogging Tendencies
Electronegativity measures an atom's ability to attract electrons in a chemical bond. It generally decreases as you move down a group because the outer electrons are further from the nucleus and less influenced by its positive charge. Moving across a period, electronegativity typically increases due to the increasing nuclear charge.
- Example: Fluorine (F) has the highest electronegativity, strongly attracting electrons in bonds, whereas Francium (Fr) has the lowest.
4. Electron Affinity: Accepting Electrons
Electron affinity is the energy change when an electron is added to a neutral atom. While less predictable than other trends, it generally shows some patterns. For instance, within a group, electron affinity may fluctuate but tends to decrease as you move down due to increasing atomic size and shielding effects.
- Example: The halogens (Group 17) generally have high electron affinities, readily accepting electrons to complete their octet, while alkali metals have lower electron affinities.
5. Metallic Character: A Spectrum of Properties
Metallic character refers to the properties typically associated with metals, such as conductivity, malleability, and ductility. It generally increases as you move down a group and decreases across a period. This trend is linked to the ease of losing electrons, a characteristic more pronounced in larger atoms further from the nucleus.
- Example: Francium exhibits strong metallic character, while Fluorine is a nonmetal.
Specific Element Family Examples: Illustrating the Similarities
Let's delve into specific element families to highlight the striking similarities in their properties:
Alkali Metals (Group 1): The Reactive Rebels
Alkali metals, including Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), and Francium (Fr), are highly reactive due to their single valence electron. They readily lose this electron to form +1 ions, making them excellent reducing agents. They are soft, silvery-white metals, and react violently with water, producing hydrogen gas.
Alkaline Earth Metals (Group 2): Slightly Less Reactive
Similar to alkali metals, alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra) have two valence electrons, but their reactivity is less pronounced. They tend to lose two electrons to form +2 ions. They are harder and denser than alkali metals but still exhibit characteristic metallic properties.
Halogens (Group 17): The Electron Grabbers
Halogens (F, Cl, Br, I, At) have seven valence electrons and are highly reactive nonmetals. They readily gain one electron to form -1 ions, achieving a stable octet. They are typically diatomic molecules (e.g., Cl2, F2) and are strong oxidizing agents.
Noble Gases (Group 18): The Inert Ones
Noble gases (He, Ne, Ar, Kr, Xe, Rn) possess a complete outer electron shell, making them exceptionally unreactive. Their stability arises from their full octet (or duet for Helium), negating the need to gain or lose electrons. They are all gases under standard conditions.
Applications Based on Family Similarities
The predictable properties of elements within the same family have crucial implications for numerous applications:
- Battery Technology: Alkali metals' reactivity makes them ideal for use in batteries, providing high energy density. Lithium-ion batteries are a prime example.
- Lighting: Halogens are used in various lighting applications, with their reactivity influencing the light output.
- Medical Applications: Certain halogens play critical roles as disinfectants and in medical imaging.
- Materials Science: The properties of various element families are carefully considered in the design and synthesis of new materials with specific characteristics.
Conclusion: The Power of Periodic Trends
The periodic table's organization highlights the profound influence of valence electron configuration on elemental properties. Elements within the same family exhibit similar chemical and physical characteristics due to their shared number of valence electrons. Understanding these periodic trends is not merely an academic exercise; it is a powerful tool for predicting and manipulating the behavior of matter, enabling innovation across various scientific and technological fields. The similarities within element families are the key to unlocking a vast array of applications, pushing the boundaries of human ingenuity and technological advancements. Further exploration of these trends opens doors to a deeper understanding of chemical bonding, reactivity, and material properties, ultimately contributing to a richer comprehension of the natural world.
Latest Posts
Latest Posts
-
Is Alcohol A Acid And A Base Bronsted
Mar 19, 2025
-
Do Acids Gain Or Lose Hydrogen Ions
Mar 19, 2025
-
Lewis Diagram For A Ion With A Total Of Electrons
Mar 19, 2025
-
A The Symbol For Sample Standard Deviation Is
Mar 19, 2025
-
What Are The Columns In The Periodic Table Called
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Elements In An Element Family Have Similar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
