Strong Acid And Strong Base Reaction
Muz Play
Mar 19, 2025 · 6 min read
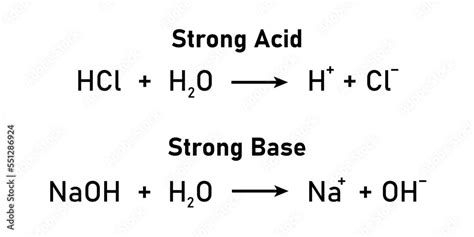
Table of Contents
Strong Acid and Strong Base Reactions: A Comprehensive Guide
Strong acids and strong bases are fundamental concepts in chemistry, crucial for understanding numerous chemical processes and applications. Their reaction, a neutralization reaction, is a highly exothermic process with predictable stoichiometry, making it a cornerstone of quantitative analysis and industrial processes. This comprehensive guide delves deep into the intricacies of strong acid-strong base reactions, exploring their characteristics, applications, and implications.
Understanding Strong Acids and Strong Bases
Before diving into their reaction, let's establish a clear understanding of what constitutes a strong acid and a strong base.
Strong Acids: Complete Dissociation
A strong acid is an acid that completely dissociates in an aqueous solution, meaning it releases all its protons (H⁺ ions) into the solution. This complete dissociation is a defining characteristic, resulting in a high concentration of H⁺ ions and a low pH. Common examples of strong acids include:
- Hydrochloric acid (HCl): A highly corrosive acid used in various industrial processes and laboratory settings.
- Sulfuric acid (H₂SO₄): A vital industrial chemical used in the production of fertilizers, detergents, and other materials. Note that while it has two protons, it dissociates completely in its first step. The second dissociation is weaker.
- Nitric acid (HNO₃): Used extensively in the production of fertilizers and explosives.
- Perchloric acid (HClO₄): Known for its extremely strong oxidizing power and use in specialized applications.
- Hydrobromic acid (HBr): Another strong acid used in various chemical reactions.
- Hydroiodic acid (HI): Similar in properties to hydrobromic acid.
Strong Bases: Complete Dissociation
Similarly, a strong base is a base that completely dissociates in an aqueous solution, releasing all its hydroxide ions (OH⁻ ions). This leads to a high concentration of OH⁻ ions, resulting in a high pH. Examples of strong bases include:
- Sodium hydroxide (NaOH): Commonly known as lye, it's used in various industrial applications, including soap making and drain cleaning.
- Potassium hydroxide (KOH): Similar to NaOH in its properties and applications.
- Lithium hydroxide (LiOH): Used in certain applications, such as in lithium-ion batteries.
- Calcium hydroxide (Ca(OH)₂): Also known as slaked lime, it's used in construction and water treatment.
- Strontium hydroxide (Sr(OH)₂): Less common than NaOH or KOH but shares similar properties.
- Barium hydroxide (Ba(OH)₂): Another strong base with applications in specific chemical processes.
The Neutralization Reaction: A Detailed Look
The reaction between a strong acid and a strong base is a neutralization reaction. This reaction involves the combination of H⁺ ions from the acid and OH⁻ ions from the base to form water (H₂O). This is a highly exothermic process, releasing a significant amount of heat.
The general equation for the neutralization of a strong acid (HA) with a strong base (BOH) is:
HA(aq) + BOH(aq) → BA(aq) + H₂O(l)
Where:
- HA represents the strong acid.
- BOH represents the strong base.
- BA represents the salt formed.
- (aq) indicates an aqueous solution.
- (l) indicates a liquid.
Stoichiometry of the Reaction
The stoichiometry of the reaction is crucial for quantitative analysis. The balanced chemical equation provides the molar ratios between the reactants and products. For example, the neutralization of HCl with NaOH is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
This equation shows that one mole of HCl reacts with one mole of NaOH to produce one mole of NaCl (sodium chloride) and one mole of water. Understanding this stoichiometry is essential for calculating the amount of acid or base needed for complete neutralization.
Heat of Neutralization
As mentioned earlier, the neutralization of a strong acid with a strong base is highly exothermic. The heat released during this reaction is relatively constant for all strong acid-strong base combinations, approximately -57 kJ/mol. This consistency arises because the driving force of the reaction is the formation of water molecules from H⁺ and OH⁻ ions. The heat of neutralization is a useful parameter in thermochemistry and can be used to determine the enthalpy change of the reaction.
Applications of Strong Acid-Strong Base Reactions
The neutralization reaction between strong acids and strong bases has numerous practical applications across various fields:
Titration: A Quantitative Analysis Technique
Titration is a crucial analytical technique used to determine the concentration of an unknown solution. In acid-base titrations, a strong acid or base of known concentration (the titrant) is added gradually to a solution of an unknown concentration (the analyte) until the equivalence point is reached. The equivalence point is when the moles of acid and base are stoichiometrically equivalent. Indicators, which change color at or near the equivalence point, are often used to signal its arrival. This technique finds wide application in various analytical chemistry settings, including environmental monitoring, food analysis, and pharmaceutical quality control.
pH Control in Industrial Processes
Many industrial processes require precise pH control. The addition of a strong acid or base can adjust the pH to the desired level. For example, in wastewater treatment, adjusting the pH is critical for effective contaminant removal. In the chemical industry, many reactions require specific pH ranges for optimal yields, and strong acid-strong base reactions are used to maintain these conditions.
Manufacturing of Salts
The neutralization reaction also produces salts. Many salts have important commercial applications. For example, sodium chloride (NaCl) is a common salt produced by the neutralization of HCl and NaOH. This process is significant in manufacturing various products.
Factors Affecting the Reaction
Several factors influence the rate and extent of a strong acid-strong base reaction:
Concentration of Reactants
Higher concentrations of reactants lead to a faster reaction rate because more reactant molecules are available for collisions. This increases the frequency of successful collisions that lead to the formation of products.
Temperature
Increasing the temperature generally increases the reaction rate. Higher temperatures provide more kinetic energy to the reactant molecules, increasing the frequency and energy of collisions.
Presence of Catalysts
While not commonly used in strong acid-strong base reactions, catalysts can potentially increase the reaction rate by providing an alternative reaction pathway with a lower activation energy.
Safety Precautions
Strong acids and bases are highly corrosive and can cause serious injuries if handled improperly. Always wear appropriate personal protective equipment (PPE), including safety glasses, gloves, and lab coats. Work in a well-ventilated area, and follow established safety protocols when handling these chemicals. In case of accidental contact, immediately flush the affected area with copious amounts of water and seek medical attention.
Conclusion
The reaction between strong acids and strong bases is a fundamental chemical process with significant theoretical and practical implications. Understanding the complete dissociation of strong acids and bases, the stoichiometry of the neutralization reaction, and the associated heat of neutralization is crucial for various applications, including quantitative analysis, pH control in industrial processes, and salt manufacturing. Always remember to prioritize safety when working with these chemicals. Careful handling and adherence to safety protocols are essential to avoid accidents and ensure the safe and effective use of strong acids and strong bases in various chemical processes.
Latest Posts
Latest Posts
-
A The Symbol For Sample Standard Deviation Is
Mar 19, 2025
-
What Are The Columns In The Periodic Table Called
Mar 19, 2025
-
How Is The Air Volume Affected By Temperature
Mar 19, 2025
-
Difference Between Voltaic Cell And Electrolytic Cell
Mar 19, 2025
-
What Is The Classification Of Matter
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Strong Acid And Strong Base Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
