What Is The Classification Of Matter
Muz Play
Mar 19, 2025 · 6 min read
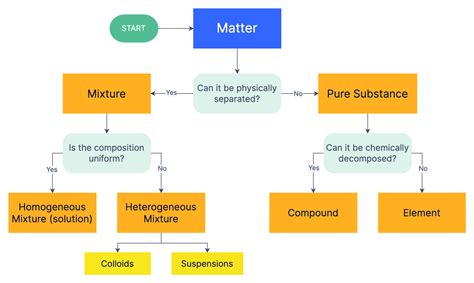
Table of Contents
What is the Classification of Matter? A Comprehensive Guide
Understanding the classification of matter is fundamental to grasping the complexities of chemistry and physics. Matter, simply defined, is anything that occupies space and has mass. This seemingly simple definition encompasses a vast array of substances, from the air we breathe to the stars in the sky. To navigate this complexity, scientists have developed various classification systems, each providing a different perspective on the fundamental building blocks of our universe. This comprehensive guide will delve into the different ways matter can be classified, exploring the properties and characteristics that distinguish one type of matter from another.
The Fundamental Classification: Pure Substances and Mixtures
The most basic classification of matter divides it into two broad categories: pure substances and mixtures. This distinction is crucial because it highlights the difference between substances with uniform composition and those with variable composition.
Pure Substances: Uniformity and Consistency
A pure substance has a fixed chemical composition throughout. This means that no matter where you sample the substance, its properties will remain consistent. Pure substances can be further divided into two categories: elements and compounds.
Elements: The Fundamental Building Blocks
Elements are the simplest form of pure substances. They are composed of only one type of atom. An atom is the smallest unit of an element that retains the chemical properties of that element. The periodic table organizes and displays all known elements, arranged by their atomic number (the number of protons in their nucleus). Examples of elements include oxygen (O), hydrogen (H), iron (Fe), and gold (Au). Elements cannot be broken down into simpler substances by chemical means.
Key Characteristics of Elements:
- Unique atomic number: Each element has a unique number of protons in its nucleus.
- Specific chemical properties: Elements exhibit specific chemical behaviors based on their electron configuration.
- Cannot be chemically decomposed: Elements are the fundamental building blocks and cannot be broken down further using chemical reactions.
Compounds: A Union of Elements
Compounds are pure substances formed when two or more elements chemically combine in a fixed ratio. This combination involves a chemical reaction that changes the properties of the constituent elements. The resulting compound has properties that are different from those of its component elements. For example, water (H₂O) is a compound formed from the combination of hydrogen and oxygen. The properties of water are drastically different from the properties of hydrogen gas and oxygen gas.
Key Characteristics of Compounds:
- Fixed ratio of elements: The elements in a compound are always present in a specific ratio.
- New properties: Compounds possess properties distinct from their constituent elements.
- Can be chemically decomposed: Compounds can be broken down into their constituent elements through chemical reactions.
Mixtures: A Blend of Substances
Unlike pure substances, mixtures contain two or more substances that are physically combined, not chemically bonded. The components of a mixture retain their individual properties, and their proportions can vary. Mixtures can be further classified into two types: homogeneous mixtures and heterogeneous mixtures.
Homogeneous Mixtures: Uniformity Throughout
Homogeneous mixtures have a uniform composition throughout. This means that the different components are evenly distributed, and the mixture appears to be a single phase. Examples include saltwater, air, and sugar dissolved in water. No matter where you take a sample from a homogeneous mixture, the composition will be the same.
Key Characteristics of Homogeneous Mixtures:
- Uniform composition: Components are evenly distributed.
- Single phase: The mixture appears uniform throughout.
- Components are not chemically bonded: Substances retain their individual properties.
Heterogeneous Mixtures: Variable Composition
Heterogeneous mixtures have a non-uniform composition. This means that the different components are not evenly distributed, and the mixture may appear to have different phases. Examples include sand and water, oil and water, and a salad. Taking samples from different parts of a heterogeneous mixture will yield different compositions.
Key Characteristics of Heterogeneous Mixtures:
- Non-uniform composition: Components are not evenly distributed.
- Multiple phases: The mixture may appear to have different regions with distinct properties.
- Components are not chemically bonded: Substances retain their individual properties.
Further Classification Based on Physical State
Matter can also be classified based on its physical state: solid, liquid, and gas. These states are determined by the arrangement and movement of the particles (atoms, molecules, or ions) that constitute the matter.
Solids: Fixed Shape and Volume
Solids have a definite shape and volume. Their particles are closely packed together in a fixed arrangement, resulting in strong intermolecular forces. Solids are generally incompressible and maintain their shape unless acted upon by an external force.
Liquids: Definite Volume, Variable Shape
Liquids have a definite volume but take the shape of their container. Their particles are closer together than in gases but more mobile than in solids. Liquids are relatively incompressible and flow easily.
Gases: Variable Shape and Volume
Gases have neither a definite shape nor a definite volume. Their particles are widely spaced and move freely, resulting in weak intermolecular forces. Gases are easily compressible and expand to fill their container.
Beyond the Basics: Plasma and Bose-Einstein Condensates
While solids, liquids, and gases are the most common states of matter encountered in everyday life, there are other states that exist under extreme conditions.
Plasma: Ionized Gas
Plasma is often referred to as the fourth state of matter. It's an ionized gas, meaning that the atoms have lost or gained electrons, creating a mixture of ions and free electrons. Plasma is highly conductive and is found in stars, lightning, and fluorescent lights.
Bose-Einstein Condensates: Supercooled Matter
Bose-Einstein condensates (BECs) are formed when a gas of bosons is cooled to temperatures very close to absolute zero (-273.15°C). At these extremely low temperatures, the atoms lose their individual identities and behave as a single entity, exhibiting macroscopic quantum phenomena.
Separating Mixtures: Techniques and Methods
The ability to separate mixtures into their constituent components is crucial in many scientific and industrial processes. Various techniques are employed depending on the type of mixture and the properties of its components.
- Filtration: Separates solids from liquids using a porous material.
- Distillation: Separates liquids based on their boiling points.
- Evaporation: Separates solids dissolved in liquids by evaporating the liquid.
- Chromatography: Separates substances based on their different affinities for a stationary and a mobile phase.
- Centrifugation: Separates substances based on their density using centrifugal force.
- Decantation: Carefully pouring off a liquid from a solid or another liquid.
- Magnetic separation: Separates magnetic materials from non-magnetic materials using a magnet.
Conclusion: A Dynamic Classification
The classification of matter is not static; it's a constantly evolving field. As our understanding of the universe deepens, new states of matter are discovered, and existing classifications are refined. However, the fundamental principles outlined in this guide provide a robust framework for understanding the diverse forms and properties of matter, from the simplest elements to the most complex mixtures and beyond. This understanding is pivotal in various scientific disciplines and technological advancements, demonstrating the fundamental importance of mastering the classification of matter.
Latest Posts
Latest Posts
-
Oh Once One Takes The Anatomy Final
Mar 20, 2025
-
Reactions That Release Energy Are Called
Mar 20, 2025
-
Electrophilic Addition Of Hbr To An Alkene
Mar 20, 2025
-
Final Electron Acceptor In Cellular Respiration
Mar 20, 2025
-
What Is A Lone Electron Pair
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Is The Classification Of Matter . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
