What Is A Lone Electron Pair
Muz Play
Mar 20, 2025 · 7 min read
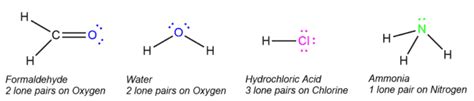
Table of Contents
What is a Lone Electron Pair? A Deep Dive into Non-Bonding Electrons
Understanding chemical bonding is crucial to grasping the fundamentals of chemistry. While we often focus on the electrons involved in bonds – the ones shared between atoms – a significant aspect often overlooked is the lone electron pair, also known as a non-bonding pair or unshared pair of electrons. This article provides a comprehensive exploration of lone electron pairs, covering their definition, impact on molecular geometry, influence on reactivity, and examples across various chemical compounds.
Defining Lone Electron Pairs: The Unshared Duet
A lone pair of electrons refers to a pair of valence electrons that are not involved in covalent bonding. These electrons are associated with a single atom and are not shared with another atom in a molecule. They occupy an atomic orbital and significantly influence the molecule's overall shape and properties. Think of them as the electrons "keeping to themselves" within the atom's electron cloud. Unlike bonding pairs, which are actively participating in creating the attractive forces holding atoms together, lone pairs exist solely within the electron shell of one atom. They are often depicted in Lewis structures as two dots, ::, placed adjacent to the atom they belong to.
Valence Electrons and Their Role
To fully appreciate lone pairs, understanding valence electrons is critical. Valence electrons are the electrons located in the outermost shell of an atom. These are the electrons involved in chemical bonding and determine the atom's reactivity. Atoms tend to gain, lose, or share valence electrons to achieve a stable electron configuration, often following the octet rule, which states that atoms tend to gain, lose, or share electrons until they are surrounded by eight valence electrons. However, exceptions exist, particularly for elements like hydrogen and lithium which aim for a duet (two electrons) and for elements in higher periods beyond the second row which can exceed the octet.
Lone pairs arise when an atom has more valence electrons than are needed to form bonds with other atoms to fulfill its octet (or duet). These extra electrons remain unshared, forming a lone pair.
The Influence of Lone Pairs on Molecular Geometry
Lone pairs exert a significant repulsive force on bonding pairs of electrons. This repulsion affects the overall molecular geometry, pushing bonded atoms further apart than they would be if only bonding pairs were present. The effect of lone pairs on the shape is more profound than that of bonding pairs because lone pairs occupy more space around the central atom due to the absence of the attractive force from another nucleus.
VSEPR Theory: A Guiding Principle
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a powerful tool for predicting the three-dimensional arrangement of atoms in a molecule. VSEPR theory postulates that electron pairs (both bonding and non-bonding) repel each other and will arrange themselves to minimize this repulsion, thus defining the molecule’s shape. The presence and number of lone pairs dictate the deviation from ideal geometries.
For example, consider methane (CH₄). Carbon has four valence electrons, each forming a single bond with a hydrogen atom. There are no lone pairs on the carbon atom, resulting in a tetrahedral geometry with bond angles of 109.5°. However, in ammonia (NH₃), nitrogen has five valence electrons, forming three bonds with hydrogen atoms and leaving one lone pair. The lone pair repels the bonding pairs, compressing the bond angles to approximately 107°. This results in a trigonal pyramidal shape, rather than the tetrahedral shape expected if only bonding pairs were considered. Water (H₂O) takes this further; oxygen has six valence electrons, forming two bonds with hydrogen and having two lone pairs. The stronger repulsion from the two lone pairs significantly compresses the bond angle to approximately 104.5°, resulting in a bent molecular geometry.
Lone Pairs and Molecular Polarity
Lone pairs play a crucial role in determining the polarity of a molecule. A polar molecule possesses a net dipole moment, meaning it has a separation of positive and negative charge. This arises from differences in electronegativity between atoms, with lone pairs enhancing this effect.
Electronegativity and Dipole Moment
Electronegativity refers to an atom's ability to attract electrons towards itself in a chemical bond. When atoms with differing electronegativities bond, the more electronegative atom will pull the shared electrons closer, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the less electronegative atom. This creates a dipole moment, represented by a vector pointing from the positive to the negative end.
Lone pairs, being localized on a single atom, contribute to the overall electron distribution within a molecule. If a molecule has a lone pair on a highly electronegative atom, the molecule is likely to be more polar because the lone pair adds to the electron density on that atom. This increased electron density enhances the partial negative charge, strengthening the dipole moment.
Lone Pairs and Chemical Reactivity
Lone pairs are not merely passive occupants of space; they actively participate in chemical reactions. Their availability to form new bonds determines the molecule's reactivity.
Acting as Lewis Bases
Lone pairs are often involved in Lewis acid-base reactions. A Lewis base is a species that donates an electron pair, while a Lewis acid accepts an electron pair. Molecules containing lone pairs readily act as Lewis bases, donating their lone pair to an electron-deficient species (Lewis acid) to form a new covalent bond. This is a fundamental aspect of many chemical reactions, including coordination chemistry and organic reactions involving nucleophiles. For instance, ammonia (NH₃) with its lone pair readily acts as a Lewis base, forming coordinate bonds with various Lewis acids.
Steric Hindrance: A Spatial Constraint
The presence of lone pairs can also influence reactivity through steric hindrance. Bulky lone pairs can physically block access to reactive sites on a molecule, reducing its reactivity or altering the reaction pathway. This effect is crucial in organic chemistry and affects reaction rates and selectivity.
Examples of Lone Pairs in Different Compounds
Let's explore some examples to illustrate the concept:
1. Water (H₂O): Oxygen has two lone pairs contributing to its bent shape and its high polarity. These lone pairs allow water to act as both a Lewis acid and a Lewis base, explaining its versatile solvent properties.
2. Ammonia (NH₃): Nitrogen has one lone pair, causing its trigonal pyramidal shape and its ability to act as a Lewis base. This lone pair is crucial for ammonia's reactivity in many chemical processes.
3. Carbonyl Compounds (e.g., Ketones, Aldehydes): The oxygen atom in carbonyl compounds possesses two lone pairs. One lone pair is involved in resonance with the carbonyl π bond, while the other is available for reactions, making these compounds susceptible to nucleophilic attack.
4. Phosphine (PH₃): Phosphorous possesses one lone pair, similar to ammonia. However, phosphine is a weaker base than ammonia due to differences in electronegativity and atomic size.
5. Sulfur Dioxide (SO₂): Sulfur has one lone pair, which, along with the double bond to one oxygen atom, contributes to its bent shape and its polar nature.
Conclusion: The Unsung Heroes of Molecular Structure and Reactivity
Lone electron pairs are fundamental components of molecules, influencing their geometry, polarity, and reactivity. While often overlooked in simplified representations, a deep understanding of their role is vital for accurately predicting molecular behavior and designing chemical reactions. By employing concepts like VSEPR theory and recognizing the impact of lone pairs on electron distribution and steric hindrance, we can unravel the complexities of chemical bonding and molecular properties. From predicting molecular shapes to understanding reactivity, lone pairs are undeniably significant in the fascinating world of chemistry. They are the unsung heroes of molecular structure and reactivity, playing a crucial role in many fundamental chemical processes. Their influence transcends simple molecular geometry; they deeply affect the overall behavior of molecules, dictating their reactivity, polarity, and interactions with other compounds. Therefore, the understanding of lone pairs is paramount for any serious study of chemistry.
Latest Posts
Latest Posts
-
Label The Parts Of The Phospholipid
Mar 20, 2025
-
Why Is Chemistry Important In The Study Of Biology
Mar 20, 2025
-
How To Determine The Equivalence Point On A Titration Curve
Mar 20, 2025
-
What Does A Flat Slope Indicate
Mar 20, 2025
-
The Path Of A Projectile Is
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Is A Lone Electron Pair . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
