Reactions That Release Energy Are Called
Muz Play
Mar 20, 2025 · 6 min read
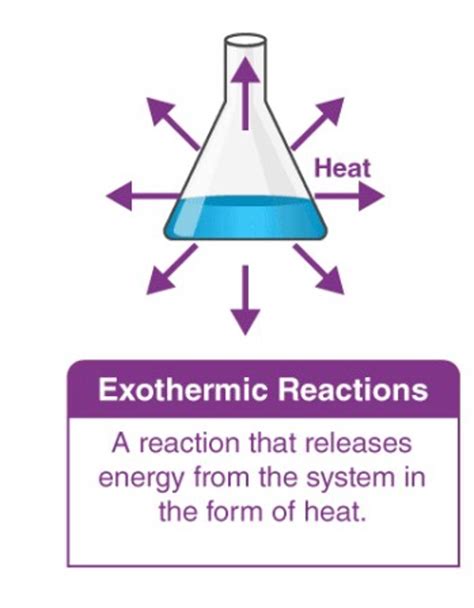
Table of Contents
Reactions That Release Energy Are Called Exothermic Reactions: A Deep Dive
Reactions that release energy are called exothermic reactions. Understanding exothermic reactions is crucial in various fields, from chemistry and physics to biology and engineering. This comprehensive guide delves into the intricacies of exothermic processes, exploring their characteristics, examples, applications, and significance in the wider world.
Understanding Exothermic Reactions: The Basics
At the heart of chemistry lies the concept of energy transfer. Chemical reactions involve the breaking and forming of chemical bonds. Some reactions absorb energy from their surroundings, while others release energy. Exothermic reactions belong to the latter category. They are characterized by the release of energy into the surrounding environment, usually in the form of heat, but sometimes also as light or sound. This energy release results in a decrease in the overall enthalpy of the system. Enthalpy (H) is a thermodynamic property representing the total heat content of a system at constant pressure. In exothermic reactions, the change in enthalpy (ΔH) is negative, signifying that the products have lower energy than the reactants.
Key Characteristics of Exothermic Reactions
-
Energy Release: The most defining characteristic is the release of energy to the surroundings. This often manifests as an increase in the temperature of the reaction mixture.
-
Negative Enthalpy Change (ΔH < 0): The enthalpy of the products is lower than the enthalpy of the reactants, indicating a net release of energy.
-
Spontaneous Reactions (Often): Many exothermic reactions occur spontaneously, although spontaneity isn't solely determined by exothermicity (entropy plays a role as well).
-
Heat Production: The heat released can be significant, sometimes leading to dramatic effects like combustion or explosions.
Examples of Exothermic Reactions: From Everyday Life to Industrial Processes
Exothermic reactions are ubiquitous, occurring in numerous everyday processes and industrial applications. Let's explore some prominent examples:
1. Combustion Reactions: The Power of Fire
Combustion is perhaps the most recognizable exothermic reaction. It involves the rapid reaction of a substance with an oxidant (usually oxygen) to produce heat and light. The burning of fuels like wood, natural gas (methane), propane, and gasoline are all classic examples of combustion. These reactions are crucial for generating energy for heating, transportation, and electricity production. The chemical equation for the combustion of methane is:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g) + Heat
The release of heat in combustion is responsible for the flames we see and the warmth we feel.
2. Neutralization Reactions: Acids and Bases Unite
Neutralization reactions between acids and bases also release heat. When an acid reacts with a base, the hydrogen ions (H⁺) from the acid combine with the hydroxide ions (OH⁻) from the base to form water. This reaction is highly exothermic, resulting in a noticeable temperature increase. For instance, the neutralization of hydrochloric acid (HCl) with sodium hydroxide (NaOH) is an exothermic process:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l) + Heat
3. Respiration: The Energy Source of Life
Cellular respiration, the process by which living organisms convert glucose and oxygen into energy, is a complex series of exothermic reactions. This process releases the chemical energy stored in glucose, providing the energy needed for various life functions. The overall reaction can be simplified as:
C₆H₁₂O₆(s) + 6O₂(g) → 6CO₂(g) + 6H₂O(l) + Energy (ATP)
The energy released is not directly as heat, but rather in the form of ATP (adenosine triphosphate), the cell's energy currency.
4. Explosions: A Violent Release of Energy
Explosions are extreme examples of exothermic reactions where a massive amount of energy is released in a very short period. The rapid expansion of gases caused by the reaction produces a powerful shockwave. Examples include the detonation of dynamite or the explosion of a bomb. These are often highly complex reactions involving various chemical species.
5. Thermite Reaction: A Spectacular Demonstration
The thermite reaction involves the reaction between a metal oxide (like iron(III) oxide) and a highly reactive metal (like aluminum). The reaction produces molten iron and a significant amount of heat, capable of melting through steel. This reaction is often used for welding and other metallurgical processes. The equation is:
Fe₂O₃(s) + 2Al(s) → 2Fe(l) + Al₂O₃(s) + Heat
Applications of Exothermic Reactions: Harnessing the Power of Heat
The energy released in exothermic reactions finds extensive applications in various fields:
1. Energy Production: Fueling Our World
Combustion of fossil fuels (coal, oil, natural gas) in power plants remains a major source of electricity. However, the environmental impact necessitates the exploration and development of cleaner energy sources.
2. Industrial Processes: Driving Chemical Transformations
Many industrial chemical processes rely on exothermic reactions. For example, the production of ammonia (Haber-Bosch process) and the manufacturing of sulfuric acid are exothermic processes.
3. Heating and Cooking: The Everyday Use of Exothermic Reactions
The burning of fuel in stoves and furnaces is a common application of exothermic reactions in our daily lives.
4. Welding and Metallurgy: Joining Metals with Heat
The thermite reaction and other exothermic processes are used for welding and other metallurgical applications, leveraging the high temperatures generated to join or modify metal structures.
Exothermic Reactions vs. Endothermic Reactions: A Comparison
Exothermic reactions are the opposite of endothermic reactions. Endothermic reactions absorb energy from their surroundings, leading to a decrease in the temperature of the reaction mixture and a positive change in enthalpy (ΔH > 0). The following table summarizes the key differences:
| Feature | Exothermic Reaction | Endothermic Reaction |
|---|---|---|
| Energy Transfer | Releases energy to surroundings | Absorbs energy from surroundings |
| ΔH | Negative (ΔH < 0) | Positive (ΔH > 0) |
| Temperature Change | Increase in temperature | Decrease in temperature |
| Spontaneity | Often spontaneous | Often non-spontaneous |
| Examples | Combustion, neutralization | Photosynthesis, melting ice |
Factors Affecting Exothermic Reactions: Optimizing Energy Release
Several factors can influence the rate and extent of energy release in exothermic reactions:
-
Concentration of Reactants: Higher concentrations generally lead to faster reactions and more significant energy release.
-
Temperature: Increasing the temperature usually accelerates the reaction rate and enhances energy release.
-
Surface Area: For reactions involving solids, a larger surface area allows for greater contact between reactants, leading to faster reactions.
-
Presence of Catalysts: Catalysts speed up reactions without being consumed themselves. They lower the activation energy required for the reaction to proceed, leading to faster energy release.
Safety Considerations: Handling Exothermic Reactions Responsibly
Exothermic reactions can be hazardous if not handled carefully. Some reactions can release substantial amounts of heat, leading to burns, fires, or explosions. Appropriate safety precautions, including protective equipment (gloves, goggles, lab coats), proper ventilation, and controlled reaction conditions, are essential when working with exothermic reactions.
Conclusion: The Importance of Exothermic Reactions
Exothermic reactions are fundamental to various aspects of our world, from powering our homes and industries to driving the processes of life itself. Understanding their characteristics, applications, and safety considerations is crucial for advancements in science, technology, and engineering. As we strive for a more sustainable future, harnessing the power of exothermic reactions responsibly is paramount. Further research and development in this field will undoubtedly lead to more innovative and efficient ways to utilize the energy released in these reactions. The ongoing exploration of exothermic reactions contributes not only to technological progress but also to our fundamental understanding of the physical and chemical world around us.
Latest Posts
Latest Posts
-
Label The Parts Of The Phospholipid
Mar 20, 2025
-
Why Is Chemistry Important In The Study Of Biology
Mar 20, 2025
-
How To Determine The Equivalence Point On A Titration Curve
Mar 20, 2025
-
What Does A Flat Slope Indicate
Mar 20, 2025
-
The Path Of A Projectile Is
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Reactions That Release Energy Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
