Difference Between Voltaic Cell And Electrolytic Cell
Muz Play
Mar 19, 2025 · 5 min read
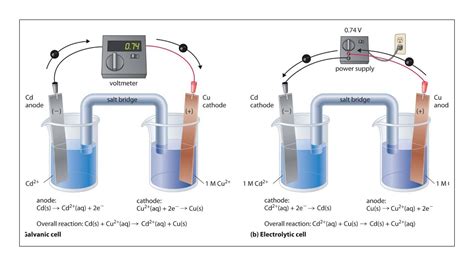
Table of Contents
Delving Deep: Voltaic Cell vs. Electrolytic Cell – A Comprehensive Comparison
Understanding the fundamental differences between voltaic cells (also known as galvanic cells) and electrolytic cells is crucial for grasping the core principles of electrochemistry. While both involve electrochemical processes, their mechanisms and applications are diametrically opposed. This comprehensive guide will explore these differences in detail, clarifying the nuances of each cell type and providing practical examples.
Defining the Key Players: Voltaic vs. Electrolytic Cells
At their core, both voltaic and electrolytic cells are systems that utilize redox reactions (reduction-oxidation reactions) to either generate or consume electrical energy. However, the direction of electron flow and the spontaneity of the reaction are what differentiate them.
Voltaic Cells: These cells are the powerhouses of electrochemistry, converting chemical energy into electrical energy spontaneously. This happens because the redox reaction within the cell is spontaneous, meaning it proceeds without any external input of electrical energy. The electrons flow spontaneously from the anode (where oxidation occurs) to the cathode (where reduction occurs), generating a potential difference that can be harnessed to power devices. Think of your everyday batteries – they are examples of voltaic cells.
Electrolytic Cells: Unlike voltaic cells, electrolytic cells consume electrical energy to drive a non-spontaneous redox reaction. An external source of electrical energy (like a battery or power supply) forces electrons to flow from the cathode (where reduction occurs) to the anode (where oxidation occurs), thus reversing the natural direction of electron flow. Electrolysis, the process of using electrical energy to drive chemical reactions, is the hallmark of electrolytic cells. This process is used extensively in various industries, from metal refining to water purification.
A Detailed Breakdown: Key Differences
Let's delve deeper into the key distinctions between these two cell types, examining them across various parameters:
1. Spontaneity of Reaction
- Voltaic Cell: The redox reaction is spontaneous; it occurs naturally without external intervention. The Gibbs Free Energy change (ΔG) is negative, indicating that the reaction releases energy.
- Electrolytic Cell: The redox reaction is non-spontaneous; it requires an external input of electrical energy to proceed. The Gibbs Free Energy change (ΔG) is positive, indicating that the reaction absorbs energy.
2. Direction of Electron Flow
- Voltaic Cell: Electrons flow spontaneously from the anode (negative electrode) to the cathode (positive electrode) through an external circuit.
- Electrolytic Cell: Electrons are forced to flow from the cathode (negative electrode) to the anode (positive electrode) by an external power source.
3. Electrode Potentials
- Voltaic Cell: The cell potential (Ecell) is positive, reflecting the spontaneous nature of the reaction. The reduction potential of the cathode is higher than the reduction potential of the anode.
- Electrolytic Cell: The cell potential (Ecell) is negative, indicating that the reaction requires external energy to proceed. The reduction potential of the cathode is lower than the reduction potential of the anode.
4. Anode and Cathode Processes
- Voltaic Cell:
- Anode: Oxidation occurs (loss of electrons). The anode is negatively charged.
- Cathode: Reduction occurs (gain of electrons). The cathode is positively charged.
- Electrolytic Cell:
- Anode: Oxidation occurs (loss of electrons). The anode is positively charged.
- Cathode: Reduction occurs (gain of electrons). The cathode is negatively charged. Note the reversal of charge compared to a voltaic cell!
5. Applications
- Voltaic Cells: Batteries (alkaline, lithium-ion, lead-acid), fuel cells, electrochemical sensors. These cells provide a portable and convenient source of electrical energy.
- Electrolytic Cells: Electroplating, electrorefining of metals (e.g., copper purification), production of chemicals (e.g., chlorine gas, sodium hydroxide), water electrolysis (producing hydrogen and oxygen). These cells are crucial in various industrial processes.
6. Cell Components
While both cell types share some basic components (electrodes, electrolyte), there are subtle differences:
- Voltaic Cell: Typically employs two different metal electrodes immersed in an electrolyte solution that allows for ion flow. A salt bridge might be present to maintain electrical neutrality.
- Electrolytic Cell: Requires an external power source connected to two electrodes immersed in an electrolyte solution. The electrodes can be made from various materials depending on the specific reaction.
Illustrative Examples: Real-World Applications
Let's examine some real-world examples to solidify our understanding:
Voltaic Cell Example: A Simple Zinc-Copper Cell
A classic example is a cell comprising a zinc electrode (anode) and a copper electrode (cathode) immersed in solutions of their respective ions (ZnSO4 and CuSO4). The spontaneous reaction involves zinc oxidizing (losing electrons) and copper ions reducing (gaining electrons). Electrons flow from the zinc electrode (anode) to the copper electrode (cathode) through an external circuit, producing a measurable voltage.
Electrolytic Cell Example: Electrolysis of Water
In the electrolysis of water, an external voltage is applied across two inert electrodes (typically platinum or graphite) immersed in water containing an electrolyte (like sulfuric acid). The applied voltage forces water molecules to decompose into hydrogen gas at the cathode (reduction) and oxygen gas at the anode (oxidation). This process is non-spontaneous and requires continuous energy input from the external source.
Beyond the Basics: Advanced Concepts
Several advanced concepts further distinguish voltaic and electrolytic cells:
- Overpotential: This refers to the extra voltage required to initiate or maintain an electrochemical reaction beyond the theoretical value predicted by thermodynamics. It is more significant in electrolytic cells where overcoming kinetic barriers is critical.
- Electrode Polarization: This phenomenon occurs when the electrode surface accumulates reaction products, hindering the reaction rate. Proper electrode design and choice of electrolyte are crucial in mitigating polarization effects in both cell types.
- Concentration Cells: A special type of voltaic cell where the driving force for electron flow is the difference in concentration of the same ion in two half-cells.
Conclusion: A Powerful Dual
Voltaic and electrolytic cells, despite their apparent similarities, represent distinct yet interconnected branches of electrochemistry. Their contrasting characteristics, stemming from the spontaneity or non-spontaneity of their redox reactions, lead to a wide array of applications, impacting various aspects of modern technology and industry. Understanding these differences is essential for anyone seeking a thorough grasp of electrochemical principles and their practical implications. Further exploration of specific cell types and applications within these categories will provide even deeper insights into this fascinating field.
Latest Posts
Latest Posts
-
The Starting Substances In A Chemical Reaction
Mar 19, 2025
-
Energy Diagram For A Two Step Reaction
Mar 19, 2025
-
Right Hand Rule For Angular Momentum
Mar 19, 2025
-
Find The Projection Of V Onto U
Mar 19, 2025
-
How Would A Structural Functionalist Explain Gender
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Voltaic Cell And Electrolytic Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
