How To Calculate Binding Energy Per Nucleon
Muz Play
Mar 23, 2025 · 5 min read
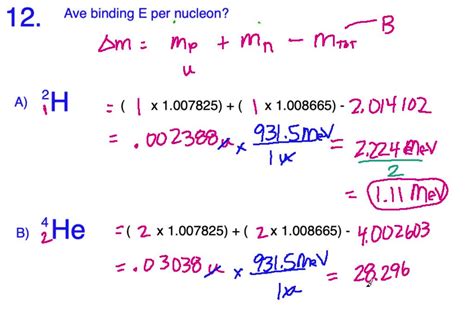
Table of Contents
How to Calculate Binding Energy Per Nucleon: A Comprehensive Guide
Understanding the binding energy per nucleon is crucial to comprehending nuclear stability and the processes that drive nuclear reactions, like nuclear fission and fusion. This quantity represents the average energy required to remove a single nucleon (proton or neutron) from a nucleus. A higher binding energy per nucleon signifies a more stable nucleus. This article will provide a comprehensive guide on how to calculate this important value, exploring the underlying concepts and providing step-by-step examples.
Understanding Binding Energy
Before diving into the calculation, let's establish a clear understanding of binding energy itself. Binding energy is the energy required to completely disassemble a nucleus into its constituent protons and neutrons. This energy is a direct measure of the strong nuclear force, the fundamental force that holds the nucleus together. It's a significant amount of energy, reflecting the immense forces at play within the atomic nucleus.
The Mass Defect: The key to understanding binding energy lies in the concept of the mass defect. The mass of a nucleus is always slightly less than the sum of the masses of its individual protons and neutrons. This difference in mass, known as the mass defect (Δm), is converted into energy according to Einstein's famous equation:
E = mc²
where:
- E is the binding energy
- m is the mass defect (in kilograms)
- c is the speed of light (approximately 3 x 10⁸ m/s)
This equation reveals the enormous energy released when a small amount of mass is converted into energy.
Calculating Binding Energy
The calculation of binding energy involves several steps:
-
Determine the number of protons and neutrons: Identify the atomic number (Z) and the mass number (A) of the nucleus. The atomic number represents the number of protons, while the mass number represents the total number of protons and neutrons (A = Z + N, where N is the number of neutrons).
-
Find the masses of protons and neutrons: Use a reliable source, such as a nuclear physics textbook or online database, to find the accurate mass of a proton (mp) and a neutron (mn). These masses are typically expressed in atomic mass units (amu). Remember that these are not simply 1 amu each; they have slightly different values.
-
Calculate the expected mass: Sum the masses of the individual protons and neutrons: Expected mass = Z * mp + N * mn
-
Determine the actual mass: Find the actual mass of the nucleus (M) from the same reliable source. This is usually expressed in amu.
-
Calculate the mass defect: Subtract the actual mass from the expected mass: Δm = (Z * mp + N * mn) - M
-
Convert the mass defect to energy: Use Einstein's equation (E = mc²) to convert the mass defect (in kilograms) to binding energy (in Joules). Remember to convert the mass defect from amu to kilograms using the conversion factor: 1 amu = 1.66054 x 10⁻²⁷ kg.
Calculating Binding Energy Per Nucleon
Once you have calculated the total binding energy, determining the binding energy per nucleon is straightforward:
Binding Energy per Nucleon = Total Binding Energy / Mass Number (A)
This value provides a crucial insight into the relative stability of the nucleus. Higher values indicate greater stability. Nuclei with mass numbers around 56 (like iron-56) exhibit the highest binding energy per nucleon, indicating exceptional stability.
Step-by-Step Example: Calculating the Binding Energy Per Nucleon of Helium-4
Let's work through a detailed example using Helium-4 (⁴He).
-
Number of protons and neutrons: Helium-4 has Z = 2 (2 protons) and A = 4 (2 protons + 2 neutrons), therefore N = 2.
-
Masses of protons and neutrons:
- Mass of a proton (mp) ≈ 1.007276 amu
- Mass of a neutron (mn) ≈ 1.008665 amu
-
Expected mass: Expected mass = (2 * 1.007276 amu) + (2 * 1.008665 amu) = 4.031882 amu
-
Actual mass: The actual mass of a Helium-4 nucleus (M) ≈ 4.001506 amu
-
Mass defect: Δm = 4.031882 amu - 4.001506 amu = 0.030376 amu
-
Convert mass defect to kilograms: Δm = 0.030376 amu * (1.66054 x 10⁻²⁷ kg/amu) ≈ 5.044 x 10⁻²⁹ kg
-
Calculate binding energy: E = mc² = (5.044 x 10⁻²⁹ kg) * (3 x 10⁸ m/s)² ≈ 4.54 x 10⁻¹² J
-
Binding energy per nucleon: Binding Energy per Nucleon = (4.54 x 10⁻¹² J) / 4 ≈ 1.135 x 10⁻¹² J/nucleon
This calculation shows that the binding energy per nucleon for Helium-4 is approximately 1.135 x 10⁻¹² Joules per nucleon. This relatively high value indicates the exceptional stability of the Helium-4 nucleus.
Applications and Significance
The calculation of binding energy per nucleon has significant applications in various fields:
- Nuclear Physics: Understanding nuclear stability and predicting the outcome of nuclear reactions.
- Nuclear Engineering: Designing nuclear reactors and weapons.
- Astrophysics: Studying stellar nucleosynthesis and the evolution of stars.
- Medical Physics: Developing radiotherapy techniques.
The concept is fundamental in explaining why certain isotopes are stable, while others undergo radioactive decay. The trend of binding energy per nucleon versus mass number reveals the energy released in both nuclear fission (splitting heavy nuclei) and nuclear fusion (combining light nuclei). These processes are vital for energy production in stars and power generation on Earth.
Advanced Considerations
While the basic calculation provides a good understanding, more advanced calculations might consider:
- Relativistic effects: At higher energies, relativistic corrections become necessary for greater accuracy.
- Nuclear shell model: This model considers the quantum mechanical structure of the nucleus, providing a more refined understanding of nuclear stability.
- Liquid drop model: This model treats the nucleus as a liquid drop, considering surface tension, Coulomb repulsion, and other factors affecting binding energy.
These advanced models provide more accurate predictions, especially for heavier nuclei where the simple mass defect calculation might show discrepancies.
Conclusion
Calculating the binding energy per nucleon is a fundamental process in nuclear physics with significant implications across multiple scientific disciplines. By understanding the underlying principles and following the steps outlined in this comprehensive guide, you can effectively determine the binding energy per nucleon for any given nucleus and gain valuable insight into its stability and the forces at play within its core. Remember to use accurate mass data from reliable sources for the most accurate results. Further exploration of advanced models can enhance the accuracy and understanding for more complex scenarios.
Latest Posts
Latest Posts
-
Identifying Reaction Types And Balancing Equations
Mar 25, 2025
-
Geography Of The Industrial Revolution Map
Mar 25, 2025
-
An Element Is Defined By The Number Of
Mar 25, 2025
-
A First Course In Differential Equations Book
Mar 25, 2025
-
Moment Of Intertia Of A Rod
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Binding Energy Per Nucleon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
