How To Calculate Standard Entropy Change
Muz Play
Mar 30, 2025 · 6 min read
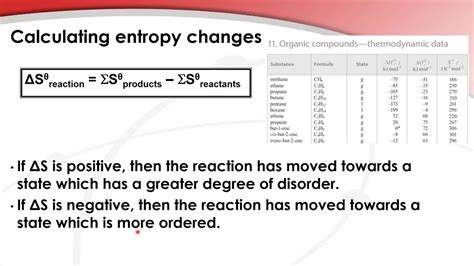
Table of Contents
How to Calculate Standard Entropy Change: A Comprehensive Guide
Standard entropy change (ΔS°) represents the change in entropy during a process under standard conditions (typically 298 K and 1 atm pressure). Understanding and calculating ΔS° is crucial in thermodynamics, providing insight into the spontaneity and feasibility of chemical reactions and physical processes. This comprehensive guide delves into the intricacies of calculating ΔS°, covering various methods and scenarios.
Understanding Entropy and its Implications
Before diving into calculations, let's establish a firm grasp of entropy itself. Entropy (S) is a thermodynamic state function that measures the randomness or disorder of a system. A system with high entropy is characterized by a large number of possible microstates or arrangements of its constituent particles. Conversely, a system with low entropy exhibits a more ordered arrangement.
The second law of thermodynamics dictates that the total entropy of an isolated system can only increase over time or remain constant in ideal cases where the system is in a steady state or undergoing a reversible process. This law underlies the concept of spontaneity. A process is spontaneous if it leads to an increase in the total entropy of the universe (system + surroundings).
The standard entropy change (ΔS°) for a reaction quantifies the change in disorder during the process under standard conditions. A positive ΔS° indicates an increase in disorder (more randomness), while a negative ΔS° signifies a decrease in disorder (more order).
Methods for Calculating Standard Entropy Change
Several methods exist for calculating ΔS°, each suited to different circumstances.
1. Using Standard Entropy Values
The most straightforward method involves utilizing tabulated standard molar entropy values (S°) for reactants and products. These values are typically found in thermodynamic data tables or handbooks. The standard entropy change is then calculated using the following equation:
ΔS°<sub>rxn</sub> = ΣnS°<sub>products</sub> - ΣmS°<sub>reactants</sub>
Where:
- ΔS°<sub>rxn</sub> is the standard entropy change of the reaction.
- n and m are the stoichiometric coefficients of the products and reactants, respectively.
- S°<sub>products</sub> and S°<sub>reactants</sub> are the standard molar entropies of the products and reactants, respectively.
Example:
Consider the combustion of methane:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g)
To calculate ΔS°, you would need the standard molar entropy values (S°) for CH₄(g), O₂(g), CO₂(g), and H₂O(g) from a thermodynamic table. Let's assume the following values (these are illustrative and may differ slightly depending on the source):
- S°(CH₄(g)) = 186.3 J/mol·K
- S°(O₂(g)) = 205.2 J/mol·K
- S°(CO₂(g)) = 213.8 J/mol·K
- S°(H₂O(g)) = 188.8 J/mol·K
ΔS°<sub>rxn</sub> = [1 × S°(CO₂(g)) + 2 × S°(H₂O(g))] - [1 × S°(CH₄(g)) + 2 × S°(O₂(g))] ΔS°<sub>rxn</sub> = [1 × 213.8 + 2 × 188.8] - [1 × 186.3 + 2 × 205.2] ΔS°<sub>rxn</sub> = 591.4 - 596.7 = -5.3 J/mol·K
This negative ΔS° indicates a decrease in disorder during the combustion of methane, which is expected as we're going from three gaseous molecules to three gaseous molecules, but with a net decrease in the number of gas particles involved.
2. Using Statistical Thermodynamics
For a more fundamental approach, statistical thermodynamics provides a way to calculate entropy from the microscopic properties of a system. This method involves considering the number of accessible microstates (Ω) for a system at a given energy. The entropy (S) is then calculated using the Boltzmann equation:
S = k<sub>B</sub> ln Ω
where:
- k<sub>B</sub> is the Boltzmann constant (1.38 × 10⁻²³ J/K).
- ln Ω is the natural logarithm of the number of microstates.
This method is particularly useful for understanding entropy at a molecular level, but it's often computationally intensive, especially for complex systems. Calculating Ω for large systems requires advanced techniques and often relies on computer simulations.
3. Calculating Entropy Changes from Heat Capacity Data
For processes involving temperature changes, the entropy change can be calculated by integrating the heat capacity (C<sub>p</sub>) over the temperature range:
ΔS = ∫(C<sub>p</sub>/T)dT
This integration requires knowing the heat capacity as a function of temperature. Often, C<sub>p</sub> is approximated as a polynomial function of temperature. This method is particularly useful when dealing with phase transitions, where there are significant changes in heat capacity. It's also helpful in cases where standard entropy values aren't readily available.
4. Using Hess's Law for Entropy Changes
Similar to its application in calculating enthalpy changes, Hess's Law can be used to determine the standard entropy change for a reaction if the standard entropy changes for intermediate reactions are known. This is particularly helpful when direct measurement of ΔS° is difficult or impossible. The principle is that the overall entropy change for a reaction is the sum of the entropy changes for the individual steps, regardless of the pathway taken.
Factors Affecting Standard Entropy Change
Several factors influence the standard entropy change of a reaction or process:
-
Phase transitions: Changes in phase (solid to liquid, liquid to gas) generally lead to significant entropy increases. Gases have much higher entropy than liquids, which in turn have higher entropy than solids.
-
Number of moles of gas: Reactions that produce a larger number of gas molecules than they consume generally exhibit a positive ΔS°. This is because gases have a much higher degree of disorder than solids or liquids.
-
Temperature: Entropy generally increases with increasing temperature, as higher temperatures provide more kinetic energy to the molecules, leading to increased randomness.
-
Molecular complexity: More complex molecules generally have higher entropy than simpler molecules due to the greater number of possible conformations and vibrational modes.
Applications of Standard Entropy Change Calculations
Calculating standard entropy change has wide-ranging applications across various fields:
- Predicting reaction spontaneity: The Gibbs free energy change (ΔG°) is related to both enthalpy (ΔH°) and entropy (ΔS°) through the equation:
ΔG° = ΔH° - TΔS°
A negative ΔG° indicates a spontaneous reaction under standard conditions. A positive ΔS° contributes to a more negative ΔG°, making a reaction more likely to be spontaneous.
-
Assessing the feasibility of chemical processes: By analyzing the entropy change, one can evaluate the likelihood of a chemical process occurring under specific conditions.
-
Understanding equilibrium constants: The equilibrium constant (K) for a reaction is related to ΔG° and therefore indirectly to ΔS°.
-
Materials science: Entropy plays a crucial role in determining the stability and properties of materials, such as alloys and polymers.
Conclusion
Calculating the standard entropy change is a fundamental aspect of thermodynamics. While the most straightforward method utilizes tabulated standard entropy values, other approaches, such as statistical thermodynamics and integration of heat capacity data, provide deeper insights into the microscopic origins of entropy changes. Understanding the factors influencing ΔS° and its applications in predicting reaction spontaneity, evaluating process feasibility, and understanding equilibrium is vital for researchers and engineers across various disciplines. By mastering these calculations, you gain a powerful tool for analyzing and predicting the behavior of chemical and physical systems.
Latest Posts
Latest Posts
-
Which Compound Is Produced During Regeneration
Apr 01, 2025
-
Periodic Trends Atomic Radius Worksheet Answers
Apr 01, 2025
-
What Is The Z Score For 98 Confidence Interval
Apr 01, 2025
-
Excessive Hormone Production Is Called Hypersecretion
Apr 01, 2025
-
What Are The Simplest Body Structures Considered Alive
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Standard Entropy Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
