How To Calculate The Binding Energy Per Nucleon
Muz Play
Mar 15, 2025 · 6 min read
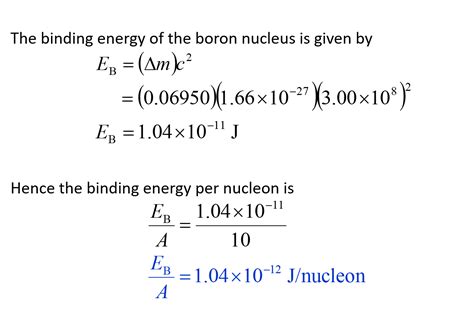
Table of Contents
How to Calculate the Binding Energy Per Nucleon: A Comprehensive Guide
Understanding the binding energy per nucleon is crucial to comprehending nuclear stability and the processes that power stars. This metric reveals the energy required to disassemble a nucleus into its constituent protons and neutrons. A higher binding energy per nucleon indicates a more stable nucleus. This article provides a comprehensive guide on how to calculate this essential quantity, delving into the underlying principles and offering practical examples.
Understanding Nuclear Binding Energy
Before diving into calculations, it's vital to grasp the concept of nuclear binding energy. This energy represents the energy required to completely separate all the nucleons (protons and neutrons) within an atomic nucleus. This energy is a direct consequence of the strong nuclear force, a fundamental force that overcomes the electrostatic repulsion between protons and holds the nucleus together.
The mass of a nucleus is always less than the sum of the masses of its individual protons and neutrons. This difference in mass, known as the mass defect, is converted into energy according to Einstein's famous equation, E=mc², where:
- E represents the binding energy
- m represents the mass defect (in kilograms)
- c represents the speed of light (approximately 3 x 10⁸ m/s)
Calculating the Mass Defect
The first step in calculating the binding energy per nucleon is determining the mass defect. This involves comparing the actual mass of the nucleus with the calculated mass of its individual components.
1. Determine the number of protons and neutrons: The atomic number (Z) represents the number of protons, while the neutron number (N) is the difference between the mass number (A) and the atomic number (A - Z).
2. Find the masses of protons and neutrons: The mass of a proton is approximately 1.007276 atomic mass units (amu), and the mass of a neutron is approximately 1.008665 amu. These values can be found in physics data tables or online resources. It's crucial to use the most accurate values available for precise calculations.
3. Calculate the expected mass: Multiply the number of protons by the mass of a proton and the number of neutrons by the mass of a neutron. Add these two values together to obtain the expected mass of the nucleus if there were no binding energy.
4. Determine the actual mass: The actual mass of the nucleus can be found in nuclear data tables. These tables provide the experimentally measured masses of various isotopes.
5. Calculate the mass defect: Subtract the actual mass of the nucleus from the expected mass. This difference represents the mass defect (Δm). Remember to use the same units (amu or kg) consistently throughout the calculation.
Calculating the Binding Energy
Once the mass defect is determined, we can calculate the binding energy using Einstein's mass-energy equivalence equation, E=mc².
1. Convert the mass defect to kilograms: If the mass defect is given in amu, it must be converted to kilograms. One amu is approximately 1.66054 x 10⁻²⁷ kg.
2. Apply Einstein's equation: Substitute the mass defect (in kg) and the speed of light (c = 3 x 10⁸ m/s) into the equation E=mc². This will yield the total binding energy of the nucleus in Joules.
3. Convert to MeV (optional): Binding energies are often expressed in mega-electronvolts (MeV). To convert from Joules to MeV, use the conversion factor: 1 MeV = 1.602 x 10⁻¹³ J.
Calculating the Binding Energy Per Nucleon
Finally, we arrive at the binding energy per nucleon, a crucial indicator of nuclear stability. This value represents the average binding energy per nucleon in the nucleus.
1. Divide the total binding energy: Divide the total binding energy (in MeV) by the total number of nucleons (A = Z + N). This calculation provides the binding energy per nucleon (MeV/nucleon).
Example Calculation: Helium-4
Let's illustrate the calculation with the helium-4 nucleus (⁴He).
1. Number of protons and neutrons: Helium-4 has 2 protons (Z = 2) and 2 neutrons (N = 2).
2. Expected mass:
- Mass of protons: 2 protons * 1.007276 amu/proton ≈ 2.014552 amu
- Mass of neutrons: 2 neutrons * 1.008665 amu/neutron ≈ 2.017330 amu
- Total expected mass: 2.014552 amu + 2.017330 amu ≈ 4.031882 amu
3. Actual mass: The actual mass of a helium-4 nucleus is approximately 4.001506 amu.
4. Mass defect:
- Δm = Expected mass - Actual mass = 4.031882 amu - 4.001506 amu ≈ 0.030376 amu
5. Mass defect in kg:
- Δm = 0.030376 amu * (1.66054 x 10⁻²⁷ kg/amu) ≈ 5.044 x 10⁻²⁹ kg
6. Binding energy (Joules):
- E = mc² = (5.044 x 10⁻²⁹ kg) * (3 x 10⁸ m/s)² ≈ 4.54 x 10⁻¹² J
7. Binding energy (MeV):
- E = (4.54 x 10⁻¹² J) / (1.602 x 10⁻¹³ J/MeV) ≈ 28.3 MeV
8. Binding energy per nucleon:
- Binding energy per nucleon = 28.3 MeV / 4 nucleons ≈ 7.075 MeV/nucleon
Therefore, the binding energy per nucleon for helium-4 is approximately 7.075 MeV/nucleon. This relatively high value contributes to the exceptional stability of the helium-4 nucleus.
Significance of Binding Energy Per Nucleon
The binding energy per nucleon is a powerful tool for understanding several key nuclear phenomena:
-
Nuclear Stability: Nuclei with higher binding energy per nucleon are more stable. The peak binding energy per nucleon occurs around iron-56, explaining its abundance in the universe.
-
Nuclear Fusion: In stars, lighter nuclei fuse together to form heavier nuclei, releasing energy because the resulting nucleus has a higher binding energy per nucleon. This process powers stars and produces elements heavier than helium.
-
Nuclear Fission: Heavier nuclei, such as uranium, can undergo fission, splitting into smaller nuclei with higher binding energy per nucleon. This process also releases a significant amount of energy, utilized in nuclear power plants and weapons.
-
Radioactive Decay: Unstable nuclei undergo radioactive decay to achieve a more stable configuration with a higher binding energy per nucleon.
Advanced Considerations
While the method outlined above provides a good approximation, several factors can influence the accuracy of the calculation:
-
Nuclear Shell Model: The shell model considers the quantum mechanical arrangement of nucleons within the nucleus. This model provides a more refined understanding of nuclear stability and can improve the accuracy of binding energy calculations.
-
Isotope Effects: Different isotopes of the same element have varying numbers of neutrons, leading to differences in their binding energies.
-
Relativistic Effects: At higher atomic numbers, relativistic effects become significant and need to be considered for accurate mass calculations.
Conclusion
Calculating the binding energy per nucleon involves a series of steps combining nuclear physics principles and Einstein's mass-energy equivalence. This value provides valuable insights into nuclear stability, fusion, fission, and radioactive decay. While the basic calculation is straightforward, more advanced models provide improved accuracy by accounting for complex nuclear phenomena. Understanding this concept is fundamental for comprehending the behavior of atomic nuclei and the energy processes governing the universe.
Latest Posts
Latest Posts
-
What Phase Of Mitosis Takes The Longest
Mar 15, 2025
-
Increased Tympanic Membrane Flexibility Older Adults
Mar 15, 2025
-
How To Calculate The Density Of A Rock
Mar 15, 2025
-
Recall That In Cellular Respiration The Processes Of Glycolysis
Mar 15, 2025
-
How Many Unpaired Electrons Are In Sulfur Atom
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate The Binding Energy Per Nucleon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
