How To Find Instantaneous Rate Of Reaction
Muz Play
Mar 18, 2025 · 7 min read
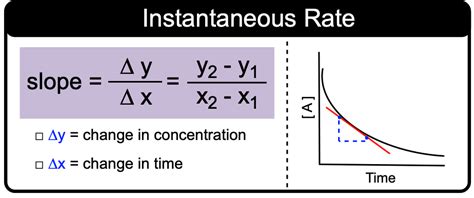
Table of Contents
How to Find the Instantaneous Rate of Reaction: A Comprehensive Guide
Determining the instantaneous rate of reaction is crucial in chemical kinetics, offering a precise understanding of reaction speed at any given moment. Unlike the average rate, which considers the overall change over a period, the instantaneous rate captures the reaction's speed at a specific point in time. This detailed guide will equip you with the knowledge and techniques to accurately calculate the instantaneous rate of reaction.
Understanding Reaction Rates
Before delving into the calculation of the instantaneous rate, it's essential to grasp the fundamental concepts of reaction rates. The rate of a chemical reaction quantifies how quickly reactants are consumed or products are formed over time. This rate is inherently dynamic; it changes as the reaction progresses due to factors like changing reactant concentrations.
Average Rate vs. Instantaneous Rate
The average rate of reaction is calculated over a finite time interval. It provides a general idea of the reaction's speed but doesn't reflect the nuances of changes in the rate during the reaction. The formula for the average rate is often expressed as:
Average Rate = Δ[concentration]/Δt
where:
- Δ[concentration] represents the change in the concentration of a reactant or product.
- Δt represents the change in time.
Conversely, the instantaneous rate of reaction provides the precise rate at a specific instant during the reaction. It's crucial for understanding the reaction's behavior at a particular point in time, giving much more detailed insights than the average rate.
Methods for Determining the Instantaneous Rate of Reaction
Several methods allow for the accurate determination of the instantaneous rate of reaction. These methods rely on understanding the relationship between concentration and time, often visualized through graphical representations.
1. Graphical Method using Concentration vs. Time Plots
The most common and intuitive approach involves plotting the concentration of a reactant or product against time. This graph visually displays the reaction's progress. The instantaneous rate at a specific time is determined by finding the slope of the tangent line to the curve at that point.
Steps:
-
Plot the data: Carefully plot the concentration (y-axis) versus time (x-axis) data obtained from experimental measurements.
-
Choose a point: Select the point on the curve that represents the specific time at which you want to determine the instantaneous rate.
-
Draw a tangent: Draw a tangent line to the curve at the chosen point. This tangent line should only touch the curve at that single point and represent the instantaneous slope. Accuracy in drawing the tangent is critical for accurate results. A ruler will greatly assist this process.
-
Calculate the slope: Determine the slope of the tangent line. The slope is calculated as the change in concentration divided by the change in time:
Instantaneous Rate = Slope = Δ[concentration]/Δt
where Δ[concentration] and Δt are determined from two distinct points on the tangent line. Note that these points are not necessarily data points from your original concentration versus time data.
Important Considerations:
- Data accuracy: The accuracy of the instantaneous rate directly depends on the quality and precision of the experimental data. More precise data points lead to more reliable slope estimations.
- Tangent accuracy: Drawing the tangent accurately is crucial. Using a ruler and carefully estimating the tangent line will enhance precision. Multiple attempts and comparisons can help to refine the drawn tangent.
- Units: Remember to include the correct units for the instantaneous rate, usually expressed as concentration per unit time (e.g., mol L⁻¹ s⁻¹, M/s).
2. Using Differential Calculus
For reactions exhibiting smoothly changing rates over time, calculus provides a more rigorous approach to finding instantaneous rates. If you have an explicit mathematical function describing the concentration as a function of time, the instantaneous rate at any time can be obtained through differentiation.
Steps:
- Obtain a concentration-time function: This function, often derived from experimental data fitting or theoretical models, expresses the concentration of a reactant or product ([A]) as a function of time (t), often denoted as [A] = f(t).
- Differentiate the function: Calculate the derivative of the concentration-time function with respect to time (df(t)/dt or d[A]/dt). This derivative represents the instantaneous rate of change of concentration at any given time.
- Substitute the time: Substitute the specific time (t) at which you want to determine the instantaneous rate into the derivative function. The resulting value is the instantaneous rate at that time.
Example:
Let's assume a reaction follows the first-order rate law: [A] = [A]₀e^(-kt), where [A]₀ is the initial concentration, k is the rate constant, and t is time.
The instantaneous rate is found by differentiating with respect to time:
d[A]/dt = -k[A]₀e^(-kt) = -k[A]
Thus, the instantaneous rate at any time 't' is directly proportional to the concentration of A at that time.
Important Considerations:
- Function accuracy: The accuracy of the calculated instantaneous rate is directly dependent on the accuracy of the concentration-time function. Using appropriate mathematical models and fitting techniques for the experimental data is crucial.
- Calculus knowledge: This method requires a sound understanding of differential calculus.
3. Numerical Differentiation Methods
When an explicit function is unavailable or impractical to obtain, numerical methods provide an alternative approach to approximate the instantaneous rate. Numerical differentiation techniques use nearby data points to approximate the derivative at a specific point. Common methods include:
- Forward difference: This method approximates the derivative using the data point at the time of interest and the next data point. It's relatively simple but can be less accurate.
- Backward difference: This uses the data point of interest and the preceding data point. Similar limitations to forward difference exist.
- Central difference: This utilizes both the preceding and succeeding data points, generally providing a more accurate approximation.
The formulas for these methods involve calculating the change in concentration divided by the change in time using neighboring data points. The central difference method, for instance, approximates the instantaneous rate as:
Instantaneous Rate ≈ ([A]<sub>t+Δt</sub> - [A]<sub>t-Δt</sub>) / 2Δt
where [A]<sub>t+Δt</sub> and [A]<sub>t-Δt</sub> are concentrations at times slightly before and after the time of interest (t), and Δt is the time interval between data points.
Important Considerations:
- Data spacing: The accuracy of numerical differentiation methods significantly depends on the spacing of the data points. More closely spaced data points generally lead to better approximations.
- Method choice: The choice of numerical differentiation method (forward, backward, or central) influences the accuracy. The central difference method usually offers superior accuracy.
Practical Applications and Significance
The ability to determine the instantaneous rate of reaction has far-reaching applications across various fields:
- Reaction mechanism studies: Determining the instantaneous rate at different stages of a reaction helps elucidate the reaction mechanism and identify rate-determining steps.
- Process optimization: In industrial processes, understanding the instantaneous rate helps optimize reaction conditions (temperature, pressure, concentration) to maximize yield and efficiency.
- Environmental monitoring: In environmental chemistry, the instantaneous rate can track the speed of pollutant degradation or formation, aiding in pollution control strategies.
- Pharmaceutical research: Studying the instantaneous rate of drug metabolism and degradation is crucial for designing effective and stable pharmaceutical formulations.
Conclusion
Determining the instantaneous rate of reaction is essential for gaining a comprehensive understanding of the dynamics of chemical reactions. The graphical method, utilizing tangent lines on concentration-time plots, offers a visually intuitive approach. For reactions described by mathematical functions, calculus provides a rigorous method for accurate calculation. Numerical differentiation provides an alternative when an explicit function is unavailable. Mastering these techniques allows researchers and scientists to analyze reaction kinetics effectively, leading to advancements in various scientific and industrial fields. Remember that the accuracy of any method depends heavily on the quality of experimental data and the careful application of the chosen technique.
Latest Posts
Latest Posts
-
Root Stems And Leaves Maintain Homeostasis
Mar 18, 2025
-
One Organism Benefits And The Other Is Unaffected
Mar 18, 2025
-
A Mixture That Forms When One Substance Dissolves Another
Mar 18, 2025
-
An Example Of An Extensive Property Of Matter Is
Mar 18, 2025
-
Spin Only Formula For Magnetic Moment
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How To Find Instantaneous Rate Of Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
