How To Find Mass Of Excess Reactant
Muz Play
Mar 23, 2025 · 6 min read
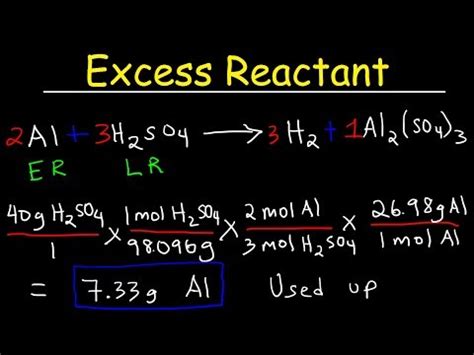
Table of Contents
How to Find the Mass of Excess Reactant: A Comprehensive Guide
Determining the mass of excess reactant is a crucial step in many stoichiometry problems in chemistry. Understanding this concept is key to mastering chemical reactions and predicting the amount of product formed. This comprehensive guide will walk you through the process step-by-step, covering various scenarios and providing practical examples to solidify your understanding.
Understanding Stoichiometry and Limiting Reactants
Before diving into calculating the mass of the excess reactant, let's refresh our understanding of stoichiometry and limiting reactants. Stoichiometry is the quantitative relationship between reactants and products in a chemical reaction. It's governed by the balanced chemical equation, which provides the molar ratios of substances involved.
The limiting reactant is the reactant that is completely consumed first in a chemical reaction. Once the limiting reactant is used up, the reaction stops, regardless of how much of the other reactants are present. The other reactants are then considered excess reactants.
Steps to Find the Mass of Excess Reactant
The process of finding the mass of excess reactant involves several key steps:
-
Balance the Chemical Equation: Ensure the chemical equation representing the reaction is balanced. This ensures the correct molar ratios between reactants and products are used in subsequent calculations. If the equation isn't balanced, your entire calculation will be incorrect.
-
Convert Grams to Moles: Convert the given masses of all reactants into moles using their respective molar masses. The molar mass of a substance is the mass of one mole of that substance, usually expressed in grams per mole (g/mol). This step is crucial because stoichiometric calculations are performed using moles, not grams.
Formula: Moles (mol) = Mass (g) / Molar Mass (g/mol)
-
Determine the Limiting Reactant: Use the mole ratios from the balanced chemical equation to determine which reactant is the limiting reactant. For each reactant, calculate the number of moles of product that would be formed if that reactant were completely consumed. The reactant that produces the least amount of product is the limiting reactant.
-
Calculate Moles of Excess Reactant Consumed: Using the mole ratio from the balanced chemical equation, determine the number of moles of the excess reactant that reacted with the limiting reactant.
-
Calculate Moles of Excess Reactant Remaining: Subtract the moles of excess reactant consumed (from step 4) from the initial moles of excess reactant (from step 2). This gives you the moles of excess reactant remaining after the reaction is complete.
-
Convert Moles to Grams: Finally, convert the moles of excess reactant remaining (from step 5) back into grams using its molar mass.
Formula: Mass (g) = Moles (mol) x Molar Mass (g/mol)
Example Problem 1: Simple Reaction
Let's consider a simple reaction between hydrogen gas (H₂) and oxygen gas (O₂) to produce water (H₂O):
2H₂(g) + O₂(g) → 2H₂O(l)
Problem: If 4 grams of hydrogen gas react with 32 grams of oxygen gas, what is the mass of the excess reactant remaining after the reaction is complete?
Solution:
-
Balanced Equation: The equation is already balanced.
-
Convert Grams to Moles:
- Moles of H₂ = 4 g / (2 g/mol) = 2 mol
- Moles of O₂ = 32 g / (32 g/mol) = 1 mol
-
Determine Limiting Reactant:
-
From the balanced equation, 2 moles of H₂ react with 1 mole of O₂.
-
If all H₂ reacts: 2 mol H₂ x (1 mol O₂ / 2 mol H₂) = 1 mol O₂ needed. We have 1 mol O₂, so O₂ is not limiting.
-
If all O₂ reacts: 1 mol O₂ x (2 mol H₂ / 1 mol O₂) = 2 mol H₂ needed. We have 2 mol H₂, so H₂ is not limiting. This means we must look further. Let's try calculating the moles of water produced by each.
-
Moles of H₂O from H₂: 2 mol H₂ x (2 mol H₂O / 2 mol H₂) = 2 mol H₂O
-
Moles of H₂O from O₂: 1 mol O₂ x (2 mol H₂O / 1 mol O₂) = 2 mol H₂O
Since both produce the same amount of water, neither reactant is in excess. This is actually a special case. Both reactants are fully consumed. The mass of the excess reactant remaining is 0g.
-
Let's modify the problem slightly to showcase an example where there is an excess reactant.
Example Problem 2: Modified Reaction with Excess Reactant
Let's assume we have 4 grams of hydrogen gas reacting with 40 grams of oxygen gas instead.
Solution:
-
Balanced Equation: The equation remains the same: 2H₂(g) + O₂(g) → 2H₂O(l)
-
Convert Grams to Moles:
- Moles of H₂ = 4 g / (2 g/mol) = 2 mol
- Moles of O₂ = 40 g / (32 g/mol) = 1.25 mol
-
Determine Limiting Reactant:
- If all H₂ reacts: 2 mol H₂ x (1 mol O₂ / 2 mol H₂) = 1 mol O₂ needed. We have 1.25 mol O₂, so H₂ is the limiting reactant.
- If all O₂ reacts: 1.25 mol O₂ x (2 mol H₂ / 1 mol O₂) = 2.5 mol H₂ needed. We only have 2 mol H₂, so O₂ is in excess.
-
Calculate Moles of Excess Reactant Consumed:
- Moles of O₂ consumed = 2 mol H₂ x (1 mol O₂ / 2 mol H₂) = 1 mol O₂
-
Calculate Moles of Excess Reactant Remaining:
- Initial moles of O₂ - moles of O₂ consumed = 1.25 mol - 1 mol = 0.25 mol O₂ remaining
-
Convert Moles to Grams:
- Mass of O₂ remaining = 0.25 mol x 32 g/mol = 8 g
Therefore, 8 grams of oxygen gas remain as the excess reactant.
Example Problem 3: More Complex Reaction
Let's consider a more complex reaction:
Fe₂O₃(s) + 3CO(g) → 2Fe(s) + 3CO₂(g)
Problem: 100g of Fe₂O₃ reacts with 50g of CO. Find the mass of the excess reactant remaining.
Solution:
-
Balanced Equation: The equation is already balanced.
-
Convert Grams to Moles:
- Molar mass Fe₂O₃ = (2 * 55.85) + (3 * 16) = 159.7 g/mol
- Moles of Fe₂O₃ = 100 g / 159.7 g/mol ≈ 0.626 mol
- Molar mass CO = 12 + 16 = 28 g/mol
- Moles of CO = 50 g / 28 g/mol ≈ 1.786 mol
-
Determine Limiting Reactant:
- From the balanced equation, 1 mole of Fe₂O₃ reacts with 3 moles of CO.
- If all Fe₂O₃ reacts: 0.626 mol Fe₂O₃ x (3 mol CO / 1 mol Fe₂O₃) ≈ 1.878 mol CO needed. We only have 1.786 mol CO, so Fe₂O₃ is not the limiting reactant; CO is limiting.
-
Calculate Moles of Excess Reactant Consumed:
- Moles of Fe₂O₃ consumed = 1.786 mol CO x (1 mol Fe₂O₃ / 3 mol CO) ≈ 0.595 mol Fe₂O₃
-
Calculate Moles of Excess Reactant Remaining:
- Initial moles of Fe₂O₃ - moles of Fe₂O₃ consumed = 0.626 mol - 0.595 mol ≈ 0.031 mol Fe₂O₃ remaining
-
Convert Moles to Grams:
- Mass of Fe₂O₃ remaining = 0.031 mol x 159.7 g/mol ≈ 4.95 g
Therefore, approximately 4.95 grams of Fe₂O₃ remain as the excess reactant.
Handling More Complex Scenarios
The principles outlined above apply even to more complex reactions involving multiple reactants and products. The key is to carefully follow each step, ensuring accuracy in calculations. Remember to always double-check your work and use a calculator that allows for sufficient significant figures.
Conclusion
Determining the mass of the excess reactant is a fundamental skill in stoichiometry. By understanding the concepts of limiting and excess reactants and systematically following the steps outlined above, you can confidently solve a wide range of stoichiometry problems. Remember to practice regularly with various examples to solidify your understanding. The more you practice, the easier and more intuitive these calculations will become. Good luck!
Latest Posts
Latest Posts
-
Bone Tissue Can Be Described As
Mar 24, 2025
-
A Two Letter Symbol From The Periodic Table
Mar 24, 2025
-
How Did The Catholic Church Respond To The Reformation
Mar 24, 2025
-
Are Metals On The Right Side Of The Periodic Table
Mar 24, 2025
-
Relation Between Potential Energy And Force
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about How To Find Mass Of Excess Reactant . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
