How To Find The Extinction Coefficient
Muz Play
Mar 30, 2025 · 6 min read
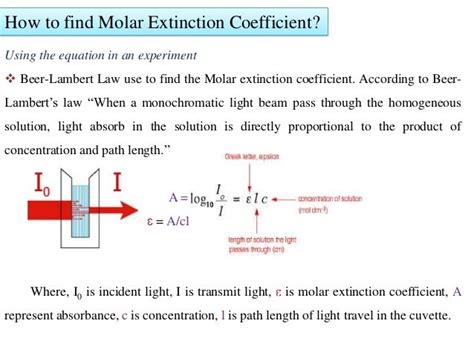
Table of Contents
How to Find the Extinction Coefficient: A Comprehensive Guide
The extinction coefficient, also known as the molar absorptivity (ε), is a crucial parameter in various fields, including chemistry, biochemistry, and environmental science. It quantifies how strongly a chemical species absorbs light at a particular wavelength. Understanding how to determine this coefficient is vital for numerous applications, from quantitative analysis to studying reaction kinetics. This comprehensive guide will delve into the methods and considerations involved in finding the extinction coefficient.
Understanding the Extinction Coefficient
Before diving into the methods, let's solidify our understanding of the extinction coefficient. It's a measure of how much light a solution absorbs at a specific wavelength. A higher extinction coefficient indicates stronger absorption. This value is specific to a given substance at a specific wavelength and solvent. The units are typically L mol⁻¹ cm⁻¹, representing the absorbance per unit concentration (mol/L) and path length (cm).
Key Factors Affecting the Extinction Coefficient:
- Wavelength: The extinction coefficient varies significantly with wavelength. It's crucial to specify the wavelength when reporting this value. A substance might have a high extinction coefficient at one wavelength and a low one at another.
- Solvent: The solvent used can influence the extinction coefficient. The interactions between the solute and solvent molecules can affect the electronic transitions responsible for light absorption.
- Temperature: Temperature can also subtly affect the extinction coefficient due to its influence on molecular interactions and conformation.
- pH: For substances that undergo protonation or deprotonation, the pH of the solution significantly impacts the extinction coefficient. Different ionic forms may exhibit distinct absorption spectra.
Methods for Determining the Extinction Coefficient
Several methods exist for determining the extinction coefficient, each with its own advantages and disadvantages. The most common approach relies on the Beer-Lambert Law.
1. Using the Beer-Lambert Law
The Beer-Lambert Law is the cornerstone of spectrophotometric analysis and provides the most straightforward method for determining the extinction coefficient. The law states:
A = εbc
Where:
- A is the absorbance (unitless)
- ε is the molar absorptivity or extinction coefficient (L mol⁻¹ cm⁻¹)
- b is the path length of the cuvette (usually 1 cm)
- c is the concentration of the analyte (mol/L)
To find the extinction coefficient using this method, you need to:
- Prepare a series of solutions: Prepare a set of solutions with known concentrations of the analyte in a suitable solvent. The concentration range should ideally span the linear region of the Beer-Lambert Law. Too high a concentration might lead to deviations from linearity.
- Measure the absorbance: Measure the absorbance of each solution at the desired wavelength using a spectrophotometer. Ensure the spectrophotometer is properly calibrated and blanked with the solvent.
- Plot the data: Plot the absorbance (A) against the concentration (c). If the Beer-Lambert Law is obeyed, the plot should be linear.
- Determine the slope: The slope of the linear plot is equal to εb. Since the path length (b) is usually 1 cm, the slope directly represents the extinction coefficient (ε).
Example: If the slope of the absorbance vs. concentration plot is 5000 L mol⁻¹ cm⁻¹, then the extinction coefficient (ε) is 5000 L mol⁻¹ cm⁻¹ at the wavelength used.
Important Considerations when using Beer-Lambert Law:
- Linearity: Ensure that the absorbance values fall within the linear range of the Beer-Lambert Law. High concentrations can lead to deviations due to intermolecular interactions.
- Solvent Choice: Choose a solvent that is transparent at the wavelength of interest and does not interact strongly with the analyte.
- Accurate Concentration Measurement: Precisely determine the concentrations of the solutions using accurate weighing and volumetric techniques. Any error in concentration directly impacts the calculated extinction coefficient.
- Temperature Control: Maintain a constant temperature during measurements as temperature can affect absorbance.
- Cuvette Cleaning: Ensure the cuvettes are clean and free of scratches to avoid scattering of light and inaccurate readings.
2. Using Literature Values
Many extinction coefficients for common compounds are already reported in scientific literature. Before undertaking experimental measurements, consult reputable databases and scientific publications to see if the extinction coefficient you need is already available. This can save significant time and effort. However, remember that the reported value may vary slightly depending on the method, solvent, and temperature used in the original study.
3. Using Computational Methods
Advanced computational methods, such as quantum mechanical calculations, can be used to predict the extinction coefficient theoretically. These methods require sophisticated software and expertise in computational chemistry. While computationally expensive, they can provide valuable insights, especially for compounds that are difficult to synthesize or measure experimentally.
Applications of the Extinction Coefficient
The extinction coefficient has numerous applications across various disciplines:
- Quantitative Analysis: It's widely used in quantitative analysis to determine the concentration of a substance in a solution by measuring its absorbance at a specific wavelength. This is used extensively in various analytical techniques like UV-Vis spectroscopy.
- Kinetic Studies: Monitoring changes in absorbance over time allows researchers to study reaction rates and mechanisms. The extinction coefficient is crucial for converting absorbance changes into concentration changes.
- Protein Quantification: In biochemistry, the extinction coefficient is used to determine the concentration of proteins. Proteins have characteristic absorption peaks in the UV region due to the aromatic amino acids (tryptophan, tyrosine, and phenylalanine).
- Environmental Monitoring: The extinction coefficient is useful in environmental monitoring to quantify the concentration of pollutants or contaminants in water or air samples.
- Material Science: In material science, the extinction coefficient helps characterize the optical properties of materials, providing valuable information for designing new materials with specific optical characteristics.
Troubleshooting Common Issues
Several issues can arise when determining the extinction coefficient. Here's a breakdown of common problems and their solutions:
- Non-linearity in Beer-Lambert Plot: This indicates a deviation from the Beer-Lambert Law, likely due to high concentrations, intermolecular interactions, or the presence of interfering substances. Dilute the solutions or choose a more suitable solvent.
- High Absorbance Values: If absorbance values are too high (typically above 1.0), it can lead to inaccuracies. Dilute the solutions and re-measure the absorbance.
- Scattering of Light: Scratched cuvettes or particulate matter in the solution can cause scattering of light, leading to erroneous absorbance readings. Ensure clean cuvettes and filter the solutions if necessary.
- Incorrect Wavelength: Ensure that you measure the absorbance at the correct wavelength, which is often the wavelength of maximum absorption (λmax).
- Instrument Calibration: Always calibrate the spectrophotometer before measurements to ensure accurate readings.
Conclusion
Determining the extinction coefficient is a fundamental technique with wide-ranging applications. The most common method utilizes the Beer-Lambert Law, requiring careful preparation of solutions, accurate measurements, and careful attention to detail. While other methods such as using literature values or computational methods exist, understanding the Beer-Lambert Law is paramount for accurate determination and interpretation of the extinction coefficient. By carefully following the outlined procedures and being mindful of the potential sources of error, researchers can obtain reliable extinction coefficient values essential for various scientific and analytical endeavors. Remember that precision and accuracy are crucial in obtaining reliable results, impacting the broader reliability of any experiment or analysis relying on this fundamental parameter.
Latest Posts
Latest Posts
-
The Presence Of A Membrane Enclosed Nucleus Is A Characteristic Of
Apr 01, 2025
-
Draw The Shear Force And Bending Moment Diagram
Apr 01, 2025
-
The Urinary System Regulates Blood Volume And Pressure By
Apr 01, 2025
-
Genomics Can Be Used In Agriculture To
Apr 01, 2025
-
What Is Used For Measuring Mass
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Extinction Coefficient . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
