How To Find The Formula Mass Of A Compound
Muz Play
Mar 15, 2025 · 6 min read
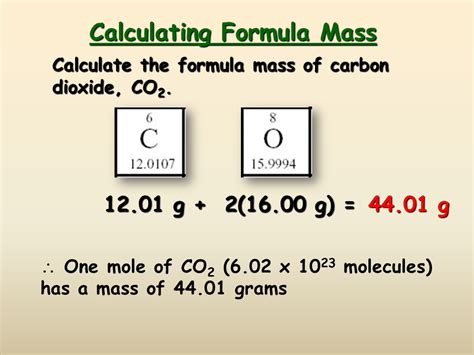
Table of Contents
How to Find the Formula Mass of a Compound: A Comprehensive Guide
Determining the formula mass of a compound is a fundamental skill in chemistry, crucial for various calculations and a deep understanding of chemical reactions. This comprehensive guide will walk you through the process, explaining the concepts, providing step-by-step instructions, and offering practical examples. We'll explore different types of compounds and tackle potential challenges you might encounter. Let's delve into the world of formula mass calculations!
Understanding Formula Mass
Before we begin the calculations, it's essential to grasp the concept of formula mass. Formula mass (also known as molecular weight for covalent compounds and formula weight for ionic compounds) represents the total mass of all the atoms present in a chemical formula. It's expressed in atomic mass units (amu) or grams per mole (g/mol). The difference between the terms "molecular weight" and "formula weight" is subtle: molecular weight refers to the mass of a molecule, while formula weight is used for ionic compounds, which exist as a lattice of ions rather than discrete molecules. However, both are calculated using the same principles.
The Importance of the Periodic Table
The periodic table is your indispensable tool for calculating formula mass. It provides the atomic mass of each element, which represents the average mass of all isotopes of that element, taking into account their relative abundance. These atomic masses are crucial for accurately calculating the formula mass of a compound.
Step-by-Step Calculation of Formula Mass
Here’s a detailed step-by-step procedure to calculate the formula mass of any compound:
Step 1: Identify the Elements and Their Number in the Chemical Formula
Carefully examine the chemical formula of the compound. Identify each element present and count the number of atoms of each element. For example, in H₂O (water), we have two hydrogen atoms (H) and one oxygen atom (O).
Step 2: Find the Atomic Mass of Each Element
Consult the periodic table to find the atomic mass of each element identified in Step 1. Remember that atomic masses are typically given to several decimal places. For our water example, the atomic mass of hydrogen (H) is approximately 1.008 amu, and the atomic mass of oxygen (O) is approximately 15.999 amu.
Step 3: Multiply the Atomic Mass by the Number of Atoms
For each element, multiply its atomic mass by the number of atoms of that element present in the chemical formula. In H₂O, we have:
- Hydrogen: 2 atoms × 1.008 amu/atom = 2.016 amu
- Oxygen: 1 atom × 15.999 amu/atom = 15.999 amu
Step 4: Add the Results Together
Finally, add the results from Step 3 to obtain the total formula mass of the compound. For water:
Formula mass (H₂O) = 2.016 amu + 15.999 amu = 18.015 amu
Therefore, the formula mass of water is approximately 18.015 amu or 18.015 g/mol.
Examples of Formula Mass Calculation
Let's practice with a few more examples:
Example 1: Sodium Chloride (NaCl)
- Elements and their numbers: 1 Sodium (Na) atom, 1 Chlorine (Cl) atom
- Atomic masses: Na (22.990 amu), Cl (35.453 amu)
- Multiplication: Na: 1 × 22.990 amu = 22.990 amu; Cl: 1 × 35.453 amu = 35.453 amu
- Addition: 22.990 amu + 35.453 amu = 58.443 amu
Formula mass of NaCl = 58.443 amu (or g/mol)
Example 2: Glucose (C₆H₁₂O₆)
- Elements and their numbers: 6 Carbon (C) atoms, 12 Hydrogen (H) atoms, 6 Oxygen (O) atoms
- Atomic masses: C (12.011 amu), H (1.008 amu), O (15.999 amu)
- Multiplication: C: 6 × 12.011 amu = 72.066 amu; H: 12 × 1.008 amu = 12.096 amu; O: 6 × 15.999 amu = 95.994 amu
- Addition: 72.066 amu + 12.096 amu + 95.994 amu = 180.156 amu
Formula mass of C₆H₁₂O₆ = 180.156 amu (or g/mol)
Example 3: Calcium Phosphate, Ca₃(PO₄)₂
This example introduces parentheses, indicating that the group within the parentheses is repeated twice.
- Elements and their numbers: 3 Calcium (Ca) atoms, 2 Phosphorus (P) atoms, 8 Oxygen (O) atoms
- Atomic masses: Ca (40.078 amu), P (30.974 amu), O (15.999 amu)
- Multiplication: Ca: 3 × 40.078 amu = 120.234 amu; P: 2 × 30.974 amu = 61.948 amu; O: 8 × 15.999 amu = 127.992 amu
- Addition: 120.234 amu + 61.948 amu + 127.992 amu = 310.174 amu
Formula mass of Ca₃(PO₄)₂ = 310.174 amu (or g/mol)
Dealing with Hydrates
Hydrates are compounds that incorporate water molecules into their crystal structure. These water molecules are indicated in the chemical formula by a dot followed by the number of water molecules. For example, copper(II) sulfate pentahydrate is written as CuSO₄·5H₂O. Calculating the formula mass of hydrates requires treating the water molecules as individual units.
Example 4: Copper(II) Sulfate Pentahydrate (CuSO₄·5H₂O)
- Elements and their numbers: 1 Copper (Cu), 1 Sulfur (S), 9 Oxygen (O), 10 Hydrogen (H)
- Atomic masses: Cu (63.546 amu), S (32.065 amu), O (15.999 amu), H (1.008 amu)
- Multiplication: Cu: 1 × 63.546 amu = 63.546 amu; S: 1 × 32.065 amu = 32.065 amu; O: 9 × 15.999 amu = 143.991 amu; H: 10 × 1.008 amu = 10.080 amu
- Addition: 63.546 amu + 32.065 amu + 143.991 amu + 10.080 amu = 249.682 amu
Formula mass of CuSO₄·5H₂O = 249.682 amu (or g/mol)
Significance of Formula Mass
Knowing the formula mass of a compound is crucial for numerous applications in chemistry, including:
- Stoichiometric calculations: Determining the amounts of reactants and products in chemical reactions.
- Molarity calculations: Calculating the concentration of solutions.
- Percent composition calculations: Determining the percentage of each element in a compound.
- Empirical and molecular formula determination: Establishing the simplest and actual chemical formulas of a compound.
Addressing Potential Challenges
While calculating formula mass is generally straightforward, some challenges might arise:
- Incorrect identification of elements or their numbers: Double-check the chemical formula carefully.
- Using incorrect atomic masses: Always refer to a reliable periodic table. Using outdated or inaccurate values will lead to errors.
- Errors in arithmetic calculations: Use a calculator and carefully review your calculations to minimize errors.
Conclusion
Calculating the formula mass of a compound is a fundamental skill for any chemistry student or professional. Mastering this process will lay the groundwork for understanding more complex chemical concepts and calculations. By following the step-by-step procedure outlined in this guide and practicing with various examples, you can confidently determine the formula mass of any compound you encounter. Remember to always double-check your work and refer to a reliable periodic table for accurate atomic masses. With practice, you'll become proficient in this essential chemical calculation.
Latest Posts
Latest Posts
-
How To Calculate The Density Of A Rock
Mar 15, 2025
-
Recall That In Cellular Respiration The Processes Of Glycolysis
Mar 15, 2025
-
How Many Unpaired Electrons Are In Sulfur Atom
Mar 15, 2025
-
Similarities Between The Romanticism And Transcendentalism Movement
Mar 15, 2025
-
Made Up Of Two Glucose Polysaccharides Amylose And Amylopectin
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Formula Mass Of A Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
