How To Find The Heat Capacity Of A Calorimeter
Muz Play
Mar 30, 2025 · 7 min read
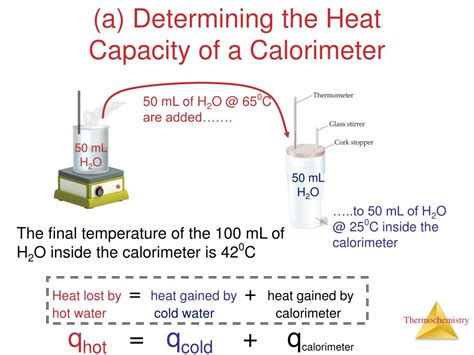
Table of Contents
How to Find the Heat Capacity of a Calorimeter: A Comprehensive Guide
Determining the heat capacity of a calorimeter, also known as the calorimeter constant, is a crucial first step in any calorimetry experiment. This constant represents the amount of heat required to raise the calorimeter's temperature by one degree Celsius (or one Kelvin). An inaccurate heat capacity measurement will lead to significant errors in subsequent enthalpy calculations. This comprehensive guide will walk you through various methods for determining this essential value.
Understanding Heat Capacity and Calorimetry
Before diving into the methods, let's establish a solid understanding of the fundamental principles.
What is Heat Capacity?
Heat capacity (C) is the amount of heat energy required to raise the temperature of a substance by one unit of temperature. It's expressed in units of J/°C or J/K. The heat capacity of a calorimeter encompasses the heat absorbed by the calorimeter itself (its container, stirrer, thermometer, etc.) and is often represented as C<sub>cal</sub>.
Calorimetry Basics
Calorimetry is a technique used to measure the heat transferred during a chemical or physical process. A calorimeter is an insulated container designed to minimize heat exchange with the surroundings. By carefully measuring temperature changes within the calorimeter, we can determine the heat involved in a reaction. The fundamental principle is based on the law of conservation of energy: the heat released by one substance is equal to the heat absorbed by another (ignoring heat loss to the surroundings).
Why Determine the Heat Capacity of a Calorimeter?
The calorimeter's heat capacity is essential because it absorbs some of the heat generated or consumed in the reaction. Failing to account for this heat absorption will result in an inaccurate measurement of the reaction's enthalpy (ΔH). The heat capacity allows us to correct for the heat absorbed by the calorimeter itself, ensuring more accurate results.
Methods for Determining the Heat Capacity of a Calorimeter
Several methods exist for determining the calorimeter constant. The choice of method often depends on the type of calorimeter being used (e.g., coffee-cup calorimeter, bomb calorimeter) and the available equipment.
Method 1: Using Water of Known Temperature
This method is commonly used with simple coffee-cup calorimeters. It involves mixing a known mass of water at a higher temperature with a known mass of water at a lower temperature within the calorimeter. By measuring the final temperature, we can calculate the calorimeter's heat capacity.
Procedure:
- Measure the mass of water (m<sub>1</sub>) at a higher temperature (T<sub>1</sub>). Use a precise balance to determine the mass.
- Measure the mass of water (m<sub>2</sub>) at a lower temperature (T<sub>2</sub>). Ensure the temperature is significantly different from T<sub>1</sub>.
- Carefully pour both samples into the calorimeter. Avoid splashing or excessive heat loss.
- Stir gently and monitor the temperature continuously. Record the final temperature (T<sub>f</sub>) after thermal equilibrium is reached.
- Calculate the heat gained by the colder water (q<sub>cold</sub>): q<sub>cold</sub> = m<sub>2</sub> * c<sub>water</sub> * (T<sub>f</sub> - T<sub>2</sub>)
- Where c<sub>water</sub> is the specific heat capacity of water (approximately 4.18 J/g°C).
- Calculate the heat lost by the hotter water (q<sub>hot</sub>): q<sub>hot</sub> = m<sub>1</sub> * c<sub>water</sub> * (T<sub>1</sub> - T<sub>f</sub>)
- Calculate the heat absorbed by the calorimeter (q<sub>cal</sub>): q<sub>cal</sub> = q<sub>hot</sub> - q<sub>cold</sub>
- Determine the heat capacity of the calorimeter (C<sub>cal</sub>): C<sub>cal</sub> = q<sub>cal</sub> / (T<sub>f</sub> - T<sub>2</sub>) or C<sub>cal</sub> = q<sub>cal</sub> / (T<sub>1</sub> - T<sub>f</sub>) depending on whether the temperature is referenced to the colder or warmer water. The values should be similar.
Important Considerations:
- Minimize heat loss: Insulate the calorimeter as much as possible.
- Stir thoroughly: Ensure uniform temperature distribution within the calorimeter.
- Accurate measurements: Use precise instruments for mass and temperature measurements.
- Thermal equilibrium: Ensure the temperature remains stable before recording the final temperature.
Method 2: Using a Known Heat Source
This method uses a known heat source, such as an electric heater, to add a specific amount of heat to the calorimeter containing a known mass of water. By measuring the temperature change, the calorimeter's heat capacity can be determined.
Procedure:
- Add a known mass of water (m) to the calorimeter.
- Record the initial temperature (T<sub>i</sub>).
- Apply a known amount of heat (q) using an electric heater for a specific duration. The heat supplied (q) can be calculated using the power of the heater (in Watts) and the time (in seconds) it is switched on: q (Joules) = Power (Watts) × Time (seconds).
- Record the final temperature (T<sub>f</sub>).
- Calculate the heat absorbed by the water (q<sub>water</sub>): q<sub>water</sub> = m * c<sub>water</sub> * (T<sub>f</sub> - T<sub>i</sub>)
- Calculate the heat absorbed by the calorimeter (q<sub>cal</sub>): q<sub>cal</sub> = q - q<sub>water</sub>
- Determine the heat capacity of the calorimeter (C<sub>cal</sub>): C<sub>cal</sub> = q<sub>cal</sub> / (T<sub>f</sub> - T<sub>i</sub>)
Important Considerations:
- Precise heat input: The heat supplied should be accurately measured and controlled.
- Heat loss correction: Consider heat loss to the surroundings and apply necessary corrections. This method is more susceptible to heat loss than Method 1.
- Calibration of the heater: Ensure the power output of the heater is accurately known.
Method 3: Using a Chemical Reaction with a Known Enthalpy Change
This method utilizes a chemical reaction with a known enthalpy change (ΔH). By measuring the temperature change of the calorimeter during the reaction, the calorimeter's heat capacity can be determined. This method is particularly useful for bomb calorimeters where reactions are carried out under constant volume.
Procedure:
- Perform a reaction within the calorimeter with a known ΔH. The reaction should be complete and occur quickly to minimize heat loss. Common examples involve the neutralization of a strong acid with a strong base.
- Record the initial (T<sub>i</sub>) and final (T<sub>f</sub>) temperatures.
- Calculate the heat released (or absorbed) by the reaction (q<sub>rxn</sub>): q<sub>rxn</sub> = n * ΔH, where 'n' is the number of moles of the limiting reactant.
- Calculate the heat absorbed by the calorimeter (q<sub>cal</sub>): q<sub>cal</sub> = -q<sub>rxn</sub> (due to conservation of energy)
- Determine the heat capacity of the calorimeter (C<sub>cal</sub>): C<sub>cal</sub> = q<sub>cal</sub> / (T<sub>f</sub> - T<sub>i</sub>)
Important Considerations:
- Known enthalpy change: The enthalpy change of the chosen reaction must be accurately known.
- Complete reaction: Ensure the reaction proceeds to completion.
- Reaction time: Minimize reaction time to reduce heat loss.
Error Analysis and Minimizing Uncertainty
Accuracy in determining the calorimeter constant is crucial. Several factors can contribute to errors:
- Heat loss to the surroundings: This is a major source of error. Proper insulation and rapid temperature measurements can minimize this.
- Incomplete reaction: If the reaction doesn't go to completion, the heat released will be less than expected.
- Calibration errors: Inaccurate calibration of instruments (thermometer, balance, heater) will affect the results.
- Heat capacity of the thermometer: The thermometer itself absorbs some heat; its contribution can be small but needs to be considered for extremely accurate measurements.
- Specific heat capacity of water: The value of 4.18 J/g°C is an approximation; temperature-dependent specific heat capacity values are more accurate.
Repeating the experiment multiple times and using statistical analysis (calculating the average and standard deviation) will improve the reliability of the results and help quantify the uncertainty associated with the determined heat capacity.
Conclusion
Determining the heat capacity of a calorimeter is a vital step in accurate calorimetry. The choice of method depends on the available resources and the type of calorimeter used. Careful attention to detail, precise measurements, and an understanding of potential error sources are crucial for obtaining reliable results. By following the procedures outlined above and employing appropriate error analysis, you can accurately determine the calorimeter constant and confidently proceed with your calorimetry experiments. Remember that minimizing heat loss and ensuring thermal equilibrium are paramount for accurate results. Through careful experimentation and data analysis, you can confidently use the determined heat capacity to accurately measure the enthalpy changes of various chemical and physical processes.
Latest Posts
Latest Posts
-
Excessive Hormone Production Is Called Hypersecretion
Apr 01, 2025
-
What Are The Simplest Body Structures Considered Alive
Apr 01, 2025
-
Chi Squared Goodness Of Fit Vs Independence
Apr 01, 2025
-
What Is The Symbol For Momentum
Apr 01, 2025
-
The Shaft Of The Bone Is Called
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Heat Capacity Of A Calorimeter . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
