How To Find The Natural Abundance Of Isotopes
Muz Play
Mar 19, 2025 · 5 min read
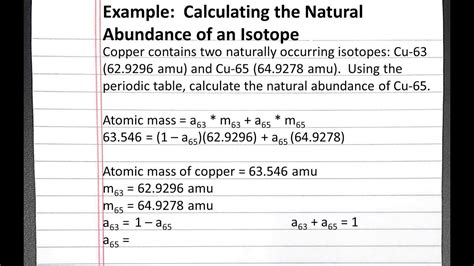
Table of Contents
- How To Find The Natural Abundance Of Isotopes
- Table of Contents
- How to Find the Natural Abundance of Isotopes
- Understanding Isotopic Abundance
- Methods for Determining Isotopic Abundance
- 1. Mass Spectrometry: The Gold Standard
- 2. Nuclear Magnetic Resonance (NMR) Spectroscopy
- 3. Atomic Emission Spectroscopy (AES)
- 4. Infrared Spectroscopy (IR)
- Theoretical Approaches & Databases
- Applications of Isotopic Abundance Data
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
How to Find the Natural Abundance of Isotopes
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This difference in neutron number results in variations in atomic mass. Understanding the natural abundance of isotopes is crucial in various scientific fields, including chemistry, physics, geology, and medicine. This article will delve into the methods used to determine the natural abundance of isotopes, exploring both experimental and theoretical approaches.
Understanding Isotopic Abundance
Before diving into the methods, let's clarify what we mean by "natural abundance." Natural abundance refers to the percentage or fraction of each isotope of an element found naturally on Earth. This percentage is typically constant for a given element, though slight variations can occur depending on the geological source of the sample. This consistency is why natural abundance is a valuable tool in various analytical techniques.
For instance, carbon has two primary stable isotopes: Carbon-12 (¹²C) and Carbon-13 (¹³C). The natural abundance of ¹²C is approximately 98.9%, while ¹³C makes up the remaining 1.1%. This seemingly small difference is significant in fields like carbon dating and isotopic analysis of organic matter.
Methods for Determining Isotopic Abundance
Several sophisticated methods are employed to determine the natural abundance of isotopes. These methods leverage the subtle differences in mass between isotopes to achieve accurate measurements.
1. Mass Spectrometry: The Gold Standard
Mass spectrometry (MS) is the most common and precise method for determining isotopic abundance. This technique ionizes a sample, accelerates the ions through a magnetic field, and separates them based on their mass-to-charge ratio (m/z). The abundance of each isotope is determined by measuring the intensity of the ion signal corresponding to each m/z value.
Different types of mass spectrometers exist, each with its own advantages and applications:
-
Gas Source Mass Spectrometry (GSMS): Suitable for analyzing gaseous samples. The sample is introduced into the mass spectrometer as a gas, ionized, and then analyzed.
-
Inductively Coupled Plasma Mass Spectrometry (ICP-MS): Excellent for analyzing liquid samples, particularly those containing metals. An inductively coupled plasma is used to ionize the sample before mass analysis.
-
Thermal Ionization Mass Spectrometry (TIMS): Known for its high precision and sensitivity, particularly useful for dating geological samples using radiogenic isotopes. The sample is thermally ionized before mass analysis.
Key Considerations in Mass Spectrometry:
-
Sample Preparation: Careful sample preparation is crucial to avoid contamination and ensure accurate results. This includes cleaning, digestion, and potentially chemical separation of the target element.
-
Calibration: Accurate calibration using certified reference materials is essential to ensure the accuracy of the measurements.
-
Interferences: Interferences from other ions with similar m/z ratios can affect the accuracy of the results. Careful consideration of potential interferences is necessary.
2. Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy is a powerful technique used to study the structure and dynamics of molecules. While not as commonly used as mass spectrometry for isotopic abundance determination, it can provide valuable information, particularly for isotopes with a nuclear spin. The technique exploits the interaction of nuclear spins with an external magnetic field. The abundance of different isotopes can be inferred from the relative intensities of the NMR signals.
Advantages of NMR:
-
Non-destructive: NMR is a non-destructive technique, meaning the sample remains intact after analysis.
-
Provides structural information: NMR provides not only isotopic abundance but also structural information about the molecule.
3. Atomic Emission Spectroscopy (AES)
Atomic emission spectroscopy is another technique that can provide information about isotopic abundance. In AES, a sample is excited, causing the atoms to emit light at specific wavelengths. The intensity of the emitted light is proportional to the concentration of the element. By analyzing the spectral lines, one can potentially infer isotopic abundance, although it's less precise than MS.
Limitations of AES:
-
Lower precision compared to MS: AES is less precise than MS for determining isotopic abundance.
-
Spectral overlaps: Spectral overlaps can make it difficult to accurately determine the abundance of specific isotopes.
4. Infrared Spectroscopy (IR)
IR spectroscopy is widely used for identifying functional groups and molecules. While not a primary method for determining isotopic abundance, IR can provide indirect evidence. The isotopic substitution of an atom can cause a shift in vibrational frequencies, which can be detected by IR spectroscopy. This shift can help infer the presence of different isotopes.
Limitations of IR for Isotopic Abundance:
-
Indirect evidence: IR provides indirect evidence, not a direct measurement of isotopic abundance.
-
Limited to specific isotopes: The isotopic shift may not be large enough to be easily detected for all isotopes.
Theoretical Approaches & Databases
While experimental techniques are crucial, theoretical calculations and established databases also play a vital role in understanding and predicting isotopic abundances.
-
Statistical Mechanics: Statistical mechanics can be used to model the distribution of isotopes in a system based on thermodynamic principles. This approach is particularly useful for predicting isotopic abundances under specific conditions.
-
Isotope Ratio Mass Spectrometry Databases: Extensive databases containing compiled isotopic ratios for various elements and materials exist. These databases serve as valuable resources for comparison and validation of experimental data.
Applications of Isotopic Abundance Data
The knowledge of isotopic abundances has far-reaching applications across numerous scientific disciplines:
-
Geochronology: Determining the age of rocks and geological formations using radiogenic isotopes.
-
Archaeology: Dating artifacts and understanding past human activities using radiocarbon dating.
-
Environmental Science: Tracing the sources of pollutants and understanding environmental processes.
-
Forensic Science: Analyzing isotopic signatures in biological samples for forensic investigations.
-
Medicine: Utilizing stable isotopes in medical imaging and metabolic studies.
-
Food Science: Analyzing the origin and authenticity of food products.
Conclusion
Determining the natural abundance of isotopes is a critical endeavor with significant implications for numerous fields. Mass spectrometry remains the gold standard due to its precision and versatility. While other techniques offer supplementary information or are suitable for specific applications, mass spectrometry's accuracy and wide applicability make it the most reliable method for determining isotopic abundance. The combination of experimental techniques, theoretical modeling, and readily available databases provides a comprehensive framework for understanding and utilizing isotopic abundance data. Further advancements in instrumentation and analytical techniques are continually refining our understanding and capabilities in this crucial area of scientific inquiry.
Latest Posts
Latest Posts
-
The Demand Curve Can Only Shift In One Direction
Mar 21, 2025
-
What Is The Mechanism Of Action Of Lipid Soluble Hormones
Mar 21, 2025
-
What Biolomecule Does Not Contian Sulfur
Mar 21, 2025
-
The Basic Unit Of Life Is The Cell
Mar 21, 2025
-
Label The Arteries Of The Head And Neck
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Natural Abundance Of Isotopes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
