How To Identify Weak And Strong Acids And Bases
Muz Play
Mar 19, 2025 · 6 min read
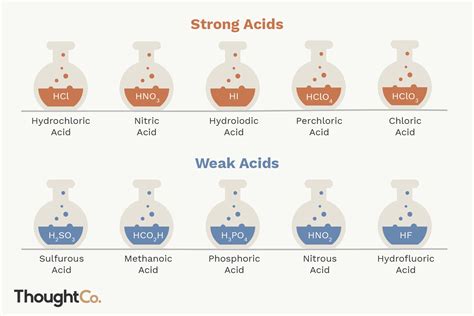
Table of Contents
How to Identify Weak and Strong Acids and Bases
Understanding the difference between weak and strong acids and bases is fundamental in chemistry. This distinction impacts numerous applications, from everyday life (like the acidity of stomach acid) to complex industrial processes. This comprehensive guide will equip you with the knowledge and tools to confidently identify weak and strong acids and bases, covering their definitions, properties, and identification methods.
Understanding Acids and Bases
Before diving into the strengths and weaknesses, let's establish a firm grasp on what acids and bases actually are. Several theories define these fundamental chemical species, but the most commonly used are the Arrhenius and Brønsted-Lowry theories.
Arrhenius Theory
The Arrhenius theory, proposed by Svante Arrhenius in the late 19th century, defines acids as substances that increase the concentration of hydrogen ions (H⁺) in an aqueous solution, and bases as substances that increase the concentration of hydroxide ions (OH⁻) in an aqueous solution. While simple, this theory has limitations; it doesn't encompass all acidic and basic substances.
Brønsted-Lowry Theory
The Brønsted-Lowry theory offers a broader perspective. It defines acids as proton donors and bases as proton acceptors. A proton, in this context, refers to a hydrogen ion (H⁺). This theory is more inclusive than the Arrhenius theory, as it accounts for acidic and basic behavior in non-aqueous solutions. For instance, ammonia (NH₃) acts as a base by accepting a proton, even though it doesn't directly produce hydroxide ions in water.
The Crucial Difference: Strength vs. Concentration
It's vital to differentiate between the strength of an acid or base and its concentration. Concentration refers to the amount of acid or base present in a solution (e.g., molarity). Strength, on the other hand, refers to the extent to which an acid or base dissociates (breaks apart) into its ions in solution.
A strong acid or base almost completely dissociates in water, meaning a large proportion of its molecules break down into ions. A weak acid or base only partially dissociates, meaning only a small percentage of its molecules break down into ions.
Identifying Strong Acids and Bases
Strong acids and bases are relatively straightforward to identify. There's a limited number of common strong acids and bases, and recognizing them usually involves memorization.
Common Strong Acids:
- Hydrochloric acid (HCl): Found in stomach acid and used in industrial cleaning.
- Sulfuric acid (H₂SO₄): A crucial industrial chemical used in various applications.
- Nitric acid (HNO₃): Used in fertilizer production and other industrial processes.
- Hydrobromic acid (HBr): Less common than HCl, H₂SO₄, and HNO₃ but still considered a strong acid.
- Hydroiodic acid (HI): Similar to HBr, it's a less frequently encountered strong acid.
- Perchloric acid (HClO₄): A powerful oxidizing agent and a very strong acid.
Common Strong Bases:
- Group 1 hydroxides (LiOH, NaOH, KOH, RbOH, CsOH): These are soluble alkali metal hydroxides that readily dissociate in water. Sodium hydroxide (NaOH), also known as lye, is particularly common.
- Group 2 hydroxides (e.g., Ca(OH)₂): While some Group 2 hydroxides are less soluble than Group 1 hydroxides, the ones that do dissolve are considered strong bases. However, their solubility significantly limits their use compared to Group 1 hydroxides.
Identifying Weak Acids and Bases
Identifying weak acids and bases is often less straightforward than identifying strong ones. There are numerous weak acids and bases, and memorization isn't as practical. Instead, we rely on other indicators and properties.
Indicators of Weak Acids and Bases:
- Incomplete Dissociation: This is the key characteristic. Weak acids and bases do not completely dissociate in water; a significant portion remains in their undissociated form.
- Low Ka/Kb Values: The acid dissociation constant (Ka) for weak acids and the base dissociation constant (Kb) for weak bases are numerically small (much less than 1). These constants quantify the extent of dissociation. A smaller Ka or Kb indicates a weaker acid or base.
- pH Values: While pH doesn't directly tell you if an acid or base is weak or strong, it gives you an indication. Strong acids have very low pH values (close to 0), while strong bases have very high pH values (close to 14). Weak acids and bases will have pH values closer to neutral (7). However, a weak acid can still have a low pH if it's highly concentrated.
- Conductivity: Strong acids and bases are good conductors of electricity because they fully dissociate into ions, which carry the electric current. Weak acids and bases are poor conductors because only a small fraction of their molecules dissociate.
Examples of Weak Acids:
- Acetic acid (CH₃COOH): Found in vinegar.
- Formic acid (HCOOH): Found in ant stings.
- Carbonic acid (H₂CO₃): Present in carbonated drinks.
- Phosphoric acid (H₃PO₄): Used in fertilizers and food additives.
- Hydrofluoric acid (HF): Although it's a weak acid, it is highly corrosive.
- Many organic acids: A vast number of organic compounds containing carboxyl groups (-COOH) are weak acids.
Examples of Weak Bases:
- Ammonia (NH₃): Used in cleaning products.
- Many amines: Organic compounds containing an amino group (-NH₂) are often weak bases.
- Pyridine (C₅H₅N): An organic nitrogenous base.
Experimental Methods for Determining Strength
While knowing the common strong acids and bases helps, experimentally determining the strength of an unknown acid or base is crucial. Several methods are available:
1. Conductivity Measurement:
Measuring the electrical conductivity of a solution provides a quick assessment. Strong acids and bases will show high conductivity, whereas weak ones will exhibit low conductivity. This method is relatively simple but not highly precise.
2. pH Measurement:
Using a pH meter provides a more accurate assessment of acidity or basicity. However, this alone doesn't definitively determine strength; concentration also plays a significant role. Combining pH measurement with other methods, such as conductivity, provides a more comprehensive analysis.
3. Titration:
Titration is a precise quantitative method to determine the concentration and strength of an acid or base. It involves reacting the unknown acid or base with a strong acid or base of known concentration. By monitoring the pH change during the titration, you can determine the equivalence point, from which you can calculate the Ka or Kb values. The resulting Ka or Kb value definitively indicates whether the acid or base is weak or strong.
Conclusion: A Holistic Approach
Identifying weak and strong acids and bases requires a holistic approach. While memorizing common strong acids and bases is helpful, understanding the concepts of dissociation, Ka/Kb values, and the experimental methods for determining acid and base strength are essential. Combining theoretical knowledge with practical techniques will allow you to confidently identify and differentiate between these crucial chemical species. Remember that concentration and strength are distinct concepts. A highly concentrated weak acid can still have a low pH, but it will not dissociate completely like a strong acid. By applying this comprehensive understanding, you can effectively navigate the world of acids and bases in chemistry.
Latest Posts
Latest Posts
-
The Metric System Is Based On Multiples Of
Mar 19, 2025
-
Failure Of Homeostatic Regulation In The Body Results In
Mar 19, 2025
-
Electric Field Of Two Point Charges
Mar 19, 2025
-
What Does A Large Equilibrium Constant Mean
Mar 19, 2025
-
Is Malleable A Chemical Or Physical Property
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How To Identify Weak And Strong Acids And Bases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
