What Does A Large Equilibrium Constant Mean
Muz Play
Mar 19, 2025 · 5 min read
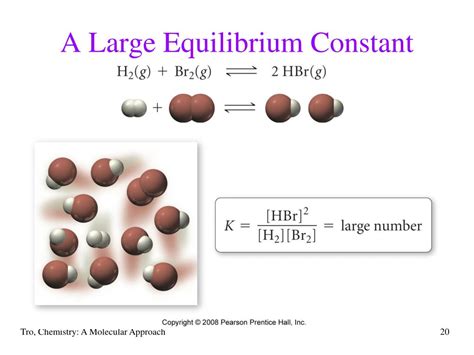
Table of Contents
What Does a Large Equilibrium Constant Mean? A Deep Dive into Chemical Equilibrium
Understanding chemical equilibrium is crucial in chemistry, and a key aspect of this understanding lies in interpreting the equilibrium constant, K. But what does it really mean when you have a large equilibrium constant? This article delves into the implications of a large K value, exploring its significance in various contexts and providing practical examples.
Understanding Equilibrium and the Equilibrium Constant (K)
Before diving into the meaning of a large K, let's refresh our understanding of chemical equilibrium. Equilibrium is the state where the rate of the forward reaction equals the rate of the reverse reaction. This doesn't mean the concentrations of reactants and products are equal; it simply means there's no net change in their concentrations over time.
The equilibrium constant, K, is a numerical value that describes the relative amounts of reactants and products at equilibrium. It's calculated using the concentrations (or partial pressures for gases) of reactants and products, raised to the power of their stoichiometric coefficients in the balanced chemical equation. For a general reversible reaction:
aA + bB ⇌ cC + dD
The equilibrium constant expression is:
K = ([C]^c[D]^d) / ([A]^a[B]^b)
Where:
- [A], [B], [C], and [D] represent the equilibrium concentrations of reactants and products.
- a, b, c, and d are the stoichiometric coefficients from the balanced equation.
The Significance of a Large Equilibrium Constant (K >> 1)
A large equilibrium constant (K >> 1, typically considered to be K > 100) signifies that at equilibrium, the concentration of products significantly outweighs the concentration of reactants. This means the reaction strongly favors the formation of products. In essence, the reaction proceeds almost to completion.
Implications of a Large K:
- High Product Yield: A large K indicates a high yield of products at equilibrium. This is highly desirable in many chemical processes, such as industrial syntheses, where maximizing product formation is crucial.
- Favorable Reaction Conditions: The reaction conditions (temperature, pressure, etc.) favor the formation of products. This might reflect the inherent thermodynamics of the reaction, with a large negative Gibbs free energy change (ΔG).
- Irreversible (or near-irreversible) Reaction Behavior: While technically all equilibrium reactions are reversible, a large K effectively makes the reaction appear irreversible under typical conditions. The reverse reaction becomes negligible compared to the forward reaction.
- Predictable Equilibrium Composition: With a large K, the equilibrium concentrations of reactants and products are easily predictable. The concentration of reactants will be very small, and the concentration of products will be close to the initial concentration of the limiting reactant.
Examples of Reactions with Large Equilibrium Constants:
Many combustion reactions exhibit exceptionally large equilibrium constants. The burning of fuels like methane (CH₄) or propane (C₃H₈) are prime examples. These reactions proceed essentially to completion under typical conditions, releasing a significant amount of energy and producing CO₂ and H₂O as the major products. The equilibrium constant for such reactions is astronomically large.
Similarly, many strong acid-base reactions have extremely large K values. The reaction between a strong acid like hydrochloric acid (HCl) and a strong base like sodium hydroxide (NaOH) produces water and salt, with a K value many orders of magnitude greater than 1.
Factors Affecting the Magnitude of the Equilibrium Constant
Several factors can influence the magnitude of the equilibrium constant:
1. Temperature:
The effect of temperature on K is governed by the enthalpy change (ΔH) of the reaction. For exothermic reactions (ΔH < 0), increasing the temperature decreases K, shifting the equilibrium towards reactants. Conversely, for endothermic reactions (ΔH > 0), increasing the temperature increases K, favoring product formation. This is described by the van't Hoff equation.
2. Pressure (for gaseous reactions):
Changes in pressure only affect the equilibrium position if the number of moles of gaseous reactants and products differs. Increasing pressure favors the side with fewer gas molecules, while decreasing pressure favors the side with more gas molecules. This is governed by Le Chatelier's principle.
3. Concentration of Reactants and Products:
Changing the concentration of reactants or products will shift the equilibrium to counteract the change, according to Le Chatelier's principle. However, this only shifts the position of equilibrium, not the value of K itself. K remains constant at a given temperature.
4. Catalysts:
Catalysts accelerate both the forward and reverse reactions equally, thereby reaching equilibrium faster. However, catalysts do not affect the value of K.
Practical Applications and Implications
The concept of a large equilibrium constant has far-reaching practical implications across various fields:
- Industrial Chemistry: Designing efficient industrial processes requires reactions with large K values to maximize product yield and minimize waste. Optimizing reaction conditions (temperature, pressure, catalysts) to achieve a large K is a major focus in chemical engineering.
- Environmental Science: Understanding equilibrium constants is crucial in predicting the fate of pollutants in the environment. For example, the equilibrium between dissolved gases and their atmospheric forms is crucial in understanding air and water pollution.
- Biochemistry and Medicine: Many biochemical reactions, such as enzyme-catalyzed reactions, have large equilibrium constants, driving the formation of essential biomolecules. Understanding these equilibrium constants is critical in understanding metabolic pathways and developing drugs.
- Analytical Chemistry: Equilibrium constants are fundamental to many analytical techniques, such as titrations and spectrophotometry, enabling the quantitative determination of substances.
Distinguishing a Large K from Fast Reaction Rates
It's crucial to distinguish between a large equilibrium constant (which indicates a favorable equilibrium position) and a fast reaction rate (which indicates how quickly equilibrium is reached). A reaction can have a large K but proceed very slowly, and vice-versa. A catalyst can speed up a reaction but won't change K. Understanding both the thermodynamic favorability (K) and the kinetic feasibility (rate) is essential for fully characterizing a chemical reaction.
Conclusion
A large equilibrium constant signifies a reaction that strongly favors the formation of products at equilibrium. This has significant implications across various scientific and engineering disciplines. Understanding the factors that influence K and the practical implications of a large K value is essential for anyone working with chemical reactions, from industrial chemists designing efficient processes to biochemists studying metabolic pathways. The value of K, combined with an understanding of reaction kinetics, provides a comprehensive picture of a chemical reaction's behavior and potential applications.
Latest Posts
Latest Posts
-
Magnetic Field From A Current Carrying Wire
Mar 19, 2025
-
Newtons Laws Of Motion Practice Problems
Mar 19, 2025
-
Lowest Methyl Numbering On A Pentane Chain
Mar 19, 2025
-
Is Naoh A Base Or Acid
Mar 19, 2025
-
Confidence Interval When Standard Deviation Is Unknown
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Does A Large Equilibrium Constant Mean . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
