Lowest Methyl Numbering On A Pentane Chain
Muz Play
Mar 19, 2025 · 6 min read
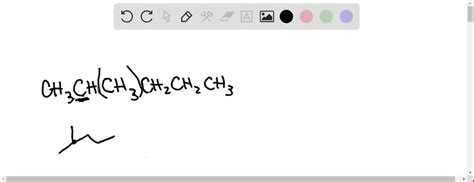
Table of Contents
Lowest Methyl Numbering on a Pentane Chain: A Comprehensive Guide to IUPAC Nomenclature
Understanding organic chemistry nomenclature, especially for branched alkanes, can be challenging. One common hurdle is determining the correct numbering of substituents, particularly methyl groups, on a pentane chain. This article will provide a thorough explanation of how to apply IUPAC rules to correctly name compounds with methyl substituents on a pentane backbone, focusing on the principle of achieving the lowest possible methyl numbering. We'll cover the fundamental rules, provide examples, and address common misconceptions to build a solid understanding of this crucial aspect of organic chemistry.
Understanding the IUPAC System
The International Union of Pure and Applied Chemistry (IUPAC) developed a systematic nomenclature system to name organic compounds. This system ensures that every organic molecule has a unique, unambiguous name, allowing for clear communication among chemists worldwide. For alkanes, the system relies on identifying the longest continuous carbon chain (the parent chain) and numbering the carbon atoms sequentially. Substituents are then named and their positions indicated by the number of the carbon atom to which they are attached.
The Importance of the Lowest Numbering
The core principle when assigning numbers to substituents is to obtain the lowest possible set of locants. This means the numbers used to describe the positions of the substituents should be as small as possible when considered as a set. Let's clarify this with an example:
Consider a pentane chain with two methyl groups. There are several ways we could potentially number this chain. However, the correct IUPAC nomenclature prioritizes the numbering system that results in the lowest numbers for the substituents.
Step-by-Step Guide to Numbering Methyl Groups on a Pentane Chain
Here's a step-by-step approach to ensure correct numbering when dealing with methyl substituents on a pentane chain:
-
Identify the Longest Carbon Chain: Locate the longest continuous carbon chain in the molecule. In the case of a pentane derivative, this will be a five-carbon chain.
-
Number the Carbon Chain: Start numbering the carbon atoms from either end of the longest chain. The goal is to assign the lowest possible numbers to the carbon atoms bearing the substituents.
-
Locate and Name the Substituents: Identify all substituents attached to the parent chain. In this case, we are primarily focusing on methyl groups (-CH₃).
-
Assign Numbers to the Substituents: Assign a number to each substituent based on the carbon atom to which it is attached. Remember that the numbers should be the lowest possible set. This is the crucial step where we prioritize the lowest numbering.
-
Arrange the Substituent Names Alphabetically: List the substituents alphabetically, irrespective of their numerical positions. However, prefixes like di, tri, tetra etc., are not considered for alphabetical ordering. This is where you'll list the methyl groups and any other substituents present.
-
Write the Complete IUPAC Name: Combine the numerical positions of the substituents, the names of the substituents, and the name of the parent chain to create the complete IUPAC name. The numbers are separated by commas, and numbers and names are separated by hyphens.
Examples Illustrating Lowest Methyl Numbering
Let's work through some examples to solidify the concept of lowest methyl numbering on a pentane chain:
Example 1:
Imagine a pentane molecule with two methyl groups on carbon atoms 2 and 3. Numbering from left to right gives us 2,3-dimethylpentane. Numbering from right to left also yields 2,3-dimethylpentane. In this case, both numbering systems give the same lowest set of numbers.
Example 2:
Consider a pentane with methyl groups on carbon atoms 2 and 4. Numbering from left to right gives 2,4-dimethylpentane. Numbering from right to left would give 2,4-dimethylpentane again.
Example 3:
Let's complicate it slightly. Consider a pentane chain with methyl groups at positions 2 and 3, and an ethyl group at position 4. Numbering from left to right gives 2,3-dimethyl-4-ethylpentane. Numbering from the other end would yield 3,4-dimethyl-2-ethylpentane; the 2,3-dimethyl-4-ethylpentane nomenclature is preferred because it gives the lowest set of numbers (2,3,4) rather than (2,3,4).
Example 4: Handling Multiple Substituents
Now, let's examine a more complex scenario with multiple methyl substituents and another type of substituent. Consider a pentane chain with three methyl groups – two on carbon 2 and one on carbon 4 – and an ethyl group on carbon 3.
-
Incorrect Numbering: Numbering from one end could lead to 2,2,4-trimethyl-3-ethylpentane. However, this is incorrect because it is not the lowest set of numbers.
-
Correct Numbering: Numbering from the opposite end gives 3-ethyl-2,2,4-trimethylpentane. While it might initially seem that there are two possible valid names, the correct name prioritizes alphabetical order of the substituents, leading to 3-ethyl-2,2,4-trimethylpentane.
Common Mistakes to Avoid
Several common mistakes can lead to incorrect naming of pentane derivatives with methyl substituents. These include:
-
Ignoring the Lowest Numbering Principle: This is the most critical error. Always strive to obtain the lowest possible set of locants when assigning numbers to substituents.
-
Incorrect Alphabetical Ordering: Remember that alphabetical order is applied after the lowest numbering has been established, and it only applies to the substituent names themselves, not the prefixes like di or tri.
-
Failing to Identify the Longest Chain: Ensure that you have correctly identified the longest continuous carbon chain before starting the numbering process.
-
Inconsistent Numbering: Maintain a consistent numbering scheme across the entire carbon chain.
Advanced Scenarios: Beyond Simple Methyl Substituents
The principles discussed above for methyl groups apply equally to other substituents on a pentane chain. The priority remains to achieve the lowest possible set of locants. For example, if you have ethyl, propyl, or even more complex substituents, the same rules for minimizing numbers and alphabetizing names apply.
Conclusion
Mastering the art of IUPAC nomenclature, particularly assigning the lowest methyl numbering on a pentane chain, is essential for any student or professional working in organic chemistry. By carefully following the steps outlined in this guide and paying close attention to detail, you can confidently assign correct IUPAC names to even complex branched alkanes, ensuring clear and unambiguous communication in the field of chemistry. Remember the key principles: find the longest chain, number for the lowest possible set of locants for all substituents, and alphabetize the substituents. Practice is crucial – working through numerous examples will help solidify your understanding and build confidence in your ability to correctly name these compounds. This comprehensive understanding will be invaluable as you progress through more advanced concepts in organic chemistry.
Latest Posts
Latest Posts
-
Is Alcohol A Acid And A Base Bronsted
Mar 19, 2025
-
Do Acids Gain Or Lose Hydrogen Ions
Mar 19, 2025
-
Lewis Diagram For A Ion With A Total Of Electrons
Mar 19, 2025
-
A The Symbol For Sample Standard Deviation Is
Mar 19, 2025
-
What Are The Columns In The Periodic Table Called
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Lowest Methyl Numbering On A Pentane Chain . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
