Is Naoh A Base Or Acid
Muz Play
Mar 19, 2025 · 5 min read
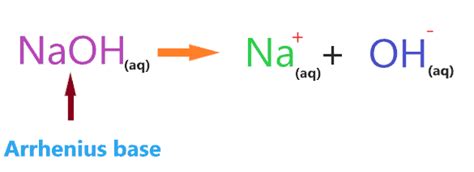
Table of Contents
Is NaOH a Base or Acid? Understanding Sodium Hydroxide's Properties
Sodium hydroxide (NaOH), also known as lye or caustic soda, is a highly alkaline substance. This means it's definitively a base, not an acid. Understanding its properties, however, goes far beyond a simple classification. This article will delve deep into the chemical nature of NaOH, exploring its behavior in aqueous solutions, its reactions, and its widespread applications, all while firmly establishing its fundamental basicity.
Defining Acids and Bases: A Quick Refresher
Before diving into the specifics of NaOH, let's briefly review the concepts of acids and bases. Several definitions exist, but the most relevant for understanding NaOH are the Arrhenius and Brønsted-Lowry definitions:
-
Arrhenius Definition: An acid is a substance that increases the concentration of hydrogen ions (H⁺) in an aqueous solution, while a base increases the concentration of hydroxide ions (OH⁻). This definition is simple but limited.
-
Brønsted-Lowry Definition: An acid is a proton (H⁺) donor, and a base is a proton acceptor. This definition is broader and encompasses more substances than the Arrhenius definition.
NaOH fits perfectly into both definitions as a base. In water, it dissociates completely, releasing hydroxide ions (OH⁻), thereby increasing the hydroxide ion concentration. It also readily accepts protons, fulfilling the Brønsted-Lowry definition of a base.
NaOH's Dissociation in Water: The Key to its Basicity
The key to understanding why NaOH is a base lies in its behavior when dissolved in water. The process is a complete dissociation:
NaOH(s) → Na⁺(aq) + OH⁻(aq)
This equation shows that when solid NaOH (s) is added to water, it breaks apart completely into sodium ions (Na⁺) and hydroxide ions (OH⁻) in an aqueous solution (aq). The presence of these hydroxide ions directly raises the pH of the solution, making it strongly alkaline. The higher the concentration of NaOH, the higher the concentration of OH⁻ and the more strongly alkaline the solution becomes.
Measuring Alkalinity: pH and pOH
The pH scale is used to measure the acidity or alkalinity of a solution. It ranges from 0 to 14, with 7 being neutral. Solutions with a pH below 7 are acidic, while those with a pH above 7 are alkaline (or basic). A solution of NaOH will have a pH significantly above 7, often approaching 14, indicating a very strong base.
The pOH scale is related to the pH scale and represents the concentration of hydroxide ions. A lower pOH indicates a higher concentration of OH⁻ and thus a stronger base. For aqueous solutions at 25°C, the relationship between pH and pOH is:
pH + pOH = 14
A highly concentrated NaOH solution will have a very low pOH and a very high pH.
Chemical Reactions of NaOH: Further Evidence of its Basic Nature
NaOH's basic nature is evident in its numerous reactions. It readily reacts with:
1. Acids: Neutralization Reactions
The most characteristic reaction of a base is its neutralization reaction with an acid. NaOH reacts with acids to form salt and water:
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
This is a classic acid-base neutralization reaction. The hydroxide ions (OH⁻) from NaOH react with the hydrogen ions (H⁺) from the acid (in this case, hydrochloric acid, HCl) to form water. The remaining ions, Na⁺ and Cl⁻, form the salt sodium chloride (NaCl). This reaction is exothermic, meaning it releases heat. Similar reactions occur with other acids like sulfuric acid (H₂SO₄) and nitric acid (HNO₃).
2. Amphoteric Substances: A Subtlety
While NaOH is predominantly a base, it's important to note that some substances, known as amphoteric substances, can act as both acids and bases depending on the context. NaOH will react with amphoteric substances like aluminum hydroxide (Al(OH)₃), exhibiting its basic nature.
3. Metals: Formation of Hydrogen Gas
NaOH reacts vigorously with some metals, particularly amphoteric metals like aluminum and zinc, generating hydrogen gas:
2NaOH(aq) + Zn(s) → Na₂ZnO₂(aq) + H₂(g)
The release of hydrogen gas is a further indicator of NaOH's reactivity as a base. Note this reaction is significantly different from the simple neutralization reactions with acids.
Applications of NaOH: A Wide Range of Uses
The strong basicity of NaOH makes it incredibly useful across numerous industries. Its applications include:
1. Industrial Cleaning: Removing Grease and Oil
NaOH's ability to dissolve fats and oils makes it an effective cleaning agent in various industrial settings. It's used in cleaning equipment, pipelines, and other industrial components.
2. Chemical Synthesis: A Crucial Reagent
NaOH is a crucial reagent in many chemical syntheses. It's used to produce soaps, detergents, paper, textiles, and a vast array of other chemicals. Its basicity plays a critical role in numerous chemical reactions.
3. Food Processing: Neutralizing Acidity
In food processing, NaOH is used to adjust the pH of foods, often to neutralize acidity. It also plays a role in processing certain foods, such as olives and pretzels.
4. Drain Cleaners: Dissolving Organic Matter
The strong base nature of NaOH makes it a common ingredient in drain cleaners. It dissolves fats, oils, and other organic matter that can clog drains. However, caution is warranted when using such products due to its corrosive nature.
Safety Precautions When Handling NaOH: A Necessary Note
NaOH is a corrosive substance. Direct contact with skin, eyes, or mucous membranes can cause severe burns and irritation. Always wear appropriate protective gear, including gloves, goggles, and a lab coat, when handling NaOH. In case of accidental contact, immediately flush the affected area with plenty of water and seek medical attention.
Conclusion: NaOH is Unequivocally a Base
In conclusion, NaOH is definitively a base, not an acid. Its complete dissociation in water, releasing hydroxide ions, its reactions with acids, its role in numerous chemical syntheses, and its widespread applications all strongly support this classification. While understanding its properties is crucial for safe and effective use, its fundamental nature as a strong base remains undisputed. Its corrosive nature necessitates careful handling, emphasizing the importance of appropriate safety precautions when working with this powerful chemical. The diverse applications of NaOH highlight its significant contribution to various industries, ranging from cleaning and manufacturing to food processing and chemical synthesis, showcasing the versatility of its basic chemical properties.
Latest Posts
Latest Posts
-
A The Symbol For Sample Standard Deviation Is
Mar 19, 2025
-
What Are The Columns In The Periodic Table Called
Mar 19, 2025
-
How Is The Air Volume Affected By Temperature
Mar 19, 2025
-
Difference Between Voltaic Cell And Electrolytic Cell
Mar 19, 2025
-
What Is The Classification Of Matter
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Naoh A Base Or Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
