How To Know If A Reaction Is Spontaneous
Muz Play
Mar 18, 2025 · 5 min read
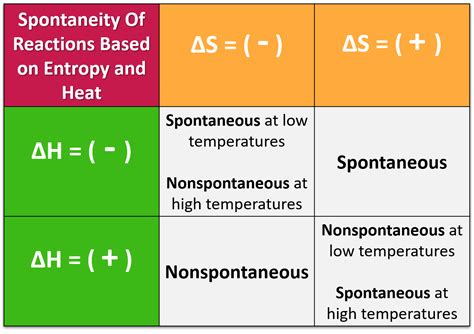
Table of Contents
How to Know if a Reaction is Spontaneous: A Comprehensive Guide
Determining whether a chemical reaction will occur spontaneously is crucial in various fields, from chemistry and engineering to biology and environmental science. Spontaneity doesn't imply speed; a spontaneous reaction might proceed slowly or rapidly. Instead, spontaneity refers to the reaction's thermodynamic favorability – its inherent tendency to proceed without external intervention. This article delves into the key concepts and methods used to predict the spontaneity of a reaction.
Understanding Spontaneity: Enthalpy, Entropy, and Gibbs Free Energy
The spontaneity of a reaction is governed by two fundamental thermodynamic properties: enthalpy (ΔH) and entropy (ΔS). These, in turn, determine the Gibbs Free Energy (ΔG), which is the ultimate predictor of spontaneity.
Enthalpy (ΔH): The Heat of Reaction
Enthalpy measures the heat absorbed or released during a reaction at constant pressure.
-
Exothermic reactions (ΔH < 0): These reactions release heat to the surroundings, often feeling warm. Exothermic reactions tend to be spontaneous because they lower the system's energy. Think of combustion – burning fuels releases heat and is spontaneous.
-
Endothermic reactions (ΔH > 0): These reactions absorb heat from the surroundings, often feeling cold. Endothermic reactions are less likely to be spontaneous because they increase the system's energy. Melting ice is an example; it requires heat input and isn't spontaneous at low temperatures.
Entropy (ΔS): The Disorder of the System
Entropy measures the randomness or disorder of a system. Reactions tend to proceed spontaneously in the direction of increased entropy.
-
Increase in entropy (ΔS > 0): This indicates an increase in disorder. Examples include the melting of a solid (more disordered liquid) or the expansion of a gas (particles spread out).
-
Decrease in entropy (ΔS < 0): This indicates a decrease in disorder. An example is the freezing of a liquid (ordered solid). Decreases in entropy are less likely to lead to spontaneous reactions.
The interplay between enthalpy and entropy is vital. A highly exothermic reaction (large negative ΔH) might be spontaneous even if it leads to a decrease in entropy (negative ΔS). Conversely, a reaction with a large increase in entropy might be spontaneous despite being slightly endothermic.
Gibbs Free Energy (ΔG): The Decisive Factor
The Gibbs Free Energy change (ΔG) combines the effects of enthalpy and entropy to predict spontaneity:
ΔG = ΔH - TΔS
where:
- ΔG is the Gibbs Free Energy change
- ΔH is the enthalpy change
- T is the temperature in Kelvin
- ΔS is the entropy change
The value of ΔG determines spontaneity under constant temperature and pressure:
-
ΔG < 0 (negative): The reaction is spontaneous under these conditions. It will proceed without external input.
-
ΔG > 0 (positive): The reaction is non-spontaneous under these conditions. It will require external input (e.g., energy) to proceed.
-
ΔG = 0 (zero): The reaction is at equilibrium; the forward and reverse reactions occur at the same rate.
Predicting Spontaneity: Practical Applications
Let's examine how to practically apply these principles to predict spontaneity.
1. Using Standard Free Energy Changes (ΔG°)
Standard free energy changes (ΔG°) are calculated under standard conditions (298 K and 1 atm pressure). These values are tabulated for many reactions and can be used as a first approximation of spontaneity. However, remember that ΔG° only indicates spontaneity under standard conditions. A reaction might be non-spontaneous under standard conditions but spontaneous under different conditions (temperature, pressure, concentration).
Calculating ΔG°:
ΔG° can be calculated using standard enthalpy changes (ΔH°) and standard entropy changes (ΔS°):
ΔG° = ΔH° - TΔS°
Standard values for ΔH° and ΔS° are readily available in thermodynamic tables.
2. Considering Temperature's Influence
Temperature plays a crucial role because it's a factor in the Gibbs Free Energy equation. The spontaneity of a reaction can change with temperature.
-
Exothermic reactions (ΔH < 0) and increasing entropy (ΔS > 0): These reactions are always spontaneous (ΔG < 0) at all temperatures.
-
Endothermic reactions (ΔH > 0) and decreasing entropy (ΔS < 0): These reactions are never spontaneous (ΔG > 0) at any temperature.
-
Exothermic reactions (ΔH < 0) and decreasing entropy (ΔS < 0): These reactions are spontaneous at low temperatures but non-spontaneous at high temperatures. At a certain temperature, the reaction will reach equilibrium (ΔG = 0).
-
Endothermic reactions (ΔH > 0) and increasing entropy (ΔS > 0): These reactions are spontaneous at high temperatures but non-spontaneous at low temperatures. A critical temperature exists at which the reaction becomes spontaneous.
3. Using Equilibrium Constants (K)
The equilibrium constant (K) is related to the standard Gibbs Free Energy change by the following equation:
ΔG° = -RTlnK
where:
- R is the ideal gas constant
- T is the temperature in Kelvin
- K is the equilibrium constant
A large K value (K >> 1) indicates a reaction that strongly favors product formation and is spontaneous under standard conditions. A small K value (K << 1) suggests the reaction strongly favors reactants and is non-spontaneous under standard conditions.
4. Qualitative Assessment of Spontaneity
Sometimes, a precise calculation of ΔG isn't necessary. A qualitative assessment based on the reaction's nature can often be sufficient:
-
Reactions leading to increased disorder (e.g., gas formation, increase in the number of molecules): These reactions are more likely to be spontaneous.
-
Reactions involving strong interactions (e.g., formation of strong bonds): These reactions are usually exothermic and thus more likely to be spontaneous.
Advanced Considerations
Non-Standard Conditions
The discussions above primarily focus on standard conditions. However, reactions often occur under non-standard conditions. To determine spontaneity under non-standard conditions, we use the following equation:
ΔG = ΔG° + RTlnQ
where Q is the reaction quotient, which reflects the current concentrations of reactants and products.
Coupled Reactions
Sometimes, non-spontaneous reactions can be driven by coupling them with highly spontaneous reactions. The overall free energy change of the coupled reactions determines the spontaneity of the entire process.
Kinetic Factors
It is crucial to remember that thermodynamics predicts spontaneity but doesn't provide information about reaction rate. A spontaneous reaction might be extremely slow if the activation energy is high. Kinetics deals with reaction rates, a separate but equally important aspect of chemical reactions.
Conclusion
Determining whether a reaction is spontaneous involves understanding the interplay between enthalpy, entropy, and temperature. The Gibbs Free Energy provides the ultimate criterion for predicting spontaneity under given conditions. By applying the principles discussed in this article and using available thermodynamic data, you can effectively predict the spontaneity of chemical reactions and understand their behavior in various systems. Remember to consider both standard and non-standard conditions, potential kinetic limitations, and the possibility of coupled reactions for a comprehensive understanding of reaction spontaneity.
Latest Posts
Latest Posts
-
How Much Energy To Be At Zero Kinetic Energy
Mar 18, 2025
-
Current As A Function Of Time
Mar 18, 2025
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
-
S P D F Blocks On The Periodic Table
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How To Know If A Reaction Is Spontaneous . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
