How To Make A Catalytic Triad
Muz Play
Mar 16, 2025 · 5 min read
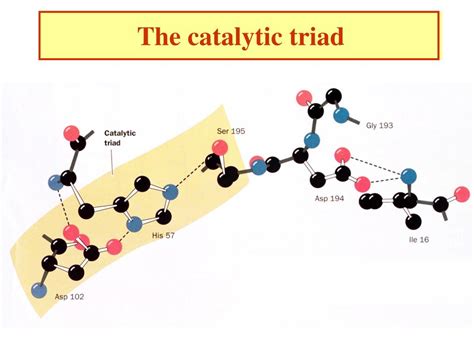
Table of Contents
How to Make a Catalytic Triad: A Deep Dive into Enzyme Engineering
Enzymes, the biological catalysts of life, often rely on a catalytic triad to perform their incredible feats of chemistry. This triad, typically composed of three amino acid residues, orchestrates precise molecular interactions to facilitate reactions that would otherwise be incredibly slow or impossible under physiological conditions. Understanding how to design and engineer catalytic triads is crucial for advancing biotechnology, drug discovery, and synthetic biology. This comprehensive guide will explore the intricacies of catalytic triads, delving into their composition, mechanisms, and the challenges and strategies involved in their creation.
Understanding the Catalytic Triad
A catalytic triad is a constellation of three amino acid residues within an enzyme's active site that work together to catalyze a reaction. These residues often cooperate through a network of hydrogen bonds and electrostatic interactions, creating a highly efficient microenvironment. While the specific residues can vary depending on the enzyme and the reaction catalyzed, some common components include:
Common Catalytic Triad Components:
- Serine (Ser): A highly versatile residue, serine's hydroxyl group acts as a nucleophile, attacking the substrate and initiating the reaction.
- Aspartate (Asp) or Glutamate (Glu): These acidic residues play a crucial role in activating the nucleophile (usually Serine) by lowering its pKa, making it more reactive. They also stabilize the transition state of the reaction.
- Histidine (His): Histidine's imidazole ring is essential for its ability to act as both an acid and a base. It acts as a general acid/base catalyst, accepting or donating protons during the reaction cycle.
Mechanistic Roles Within the Triad:
The precise roles of each residue within the triad depend on the specific enzyme and reaction, but a general mechanism can be described:
- Substrate Binding: The substrate binds to the enzyme's active site, positioning itself optimally for interaction with the catalytic triad.
- Nucleophilic Attack: The activated serine residue (due to the influence of Asp/Glu and His) performs a nucleophilic attack on the substrate, forming a covalent intermediate.
- Proton Transfer: The histidine residue acts as a general base, accepting a proton from the serine hydroxyl group.
- Substrate Transformation: The covalent intermediate undergoes further transformations, often involving the histidine as an acid catalyst.
- Product Release: The reaction products are released from the active site, regenerating the enzyme for another catalytic cycle.
Designing and Engineering Catalytic Triads: Challenges and Strategies
Creating a functional catalytic triad is a complex undertaking, involving a deep understanding of protein structure, reaction mechanisms, and computational methods. The challenges include:
Challenges:
- Spatial Arrangement: The precise spatial orientation of the three residues is paramount. Slight changes in distance or angle can drastically affect the catalytic efficiency.
- Residue Selection: Choosing the optimal residues for a specific reaction requires careful consideration of their chemical properties and reactivity.
- Protein Folding and Stability: The engineered enzyme needs to fold correctly and maintain its stability to function effectively.
- Specificity and Selectivity: The triad should exhibit high specificity for its substrate to avoid undesirable side reactions.
- Computational Demands: Designing and optimizing catalytic triads often involve computationally intensive simulations and molecular dynamics studies.
Strategies:
- Rational Design: This approach relies on detailed knowledge of the reaction mechanism and the enzyme's structure to strategically introduce or modify amino acid residues within the active site. It requires extensive computational modeling and analysis.
- Directed Evolution: This experimental method involves creating a library of enzyme variants with random mutations and selecting the most active catalysts through iterative rounds of screening and selection. It's a powerful approach for optimizing existing enzymes or creating novel ones but can be time-consuming and resource-intensive.
- Computational Design: Sophisticated computational tools, such as molecular dynamics simulations and docking studies, can be used to predict the behavior of modified enzymes and guide the design process.
- Combinatorial Approaches: Combining rational design with directed evolution can yield particularly effective results, leveraging the strengths of both approaches.
Specific Examples and Case Studies:
While detailing every example is impossible, let's explore a few illustrative cases:
Serine Proteases:
Serine proteases, such as chymotrypsin and trypsin, are well-studied enzymes that employ a catalytic triad consisting of Serine, Histidine, and Aspartate. These enzymes cleave peptide bonds by a mechanism detailed above, providing a powerful example of how a catalytic triad efficiently promotes peptide bond hydrolysis. Understanding their mechanism has greatly informed efforts to design new enzymes.
Other Enzyme Classes:
The catalytic triad concept extends beyond serine proteases. Similar triads, often with variations in the residues involved, are found in other enzyme classes, such as cysteine proteases (with a cysteine residue replacing the serine), and lipases (with a slightly modified active site architecture).
Advanced Techniques and Future Directions:
The field of catalytic triad engineering is constantly evolving, with researchers exploring new methods and strategies:
- Non-natural Amino Acids: Introducing non-natural amino acids with unique chemical properties into the catalytic triad offers opportunities to enhance catalytic activity and expand the range of reactions that can be catalyzed.
- Metal-dependent Enzymes: Integrating metal ions into the catalytic triad can enable the catalysis of reactions that require redox chemistry or other metal-mediated processes.
- Artificial Metalloenzymes: Researchers are creating artificial enzymes by incorporating metal complexes into protein scaffolds, aiming to design highly selective and efficient catalysts for a wide range of applications.
Conclusion:
Creating a functional catalytic triad requires a deep understanding of enzyme structure, function, and reaction mechanisms. While challenging, the potential rewards are significant, offering opportunities to develop new biocatalysts for various applications, including:
- Bioremediation: Engineering enzymes to degrade pollutants.
- Biofuel Production: Creating enzymes for more efficient biofuel synthesis.
- Pharmaceutical Development: Developing new drugs and therapeutics.
- Industrial Biotechnology: Creating enzymes for industrial processes.
By combining rational design, directed evolution, and advanced computational techniques, researchers are continually pushing the boundaries of enzyme engineering, leading to remarkable advances in our understanding of biological catalysis and its potential applications. The quest to understand and manipulate catalytic triads is a testament to the power of interdisciplinary research and the drive to harness nature's ingenuity for human benefit. The future promises even more sophisticated strategies and a deeper understanding of the intricate mechanisms underlying these remarkable biological machines.
Latest Posts
Latest Posts
-
Why Was The Discovery Of Noble Gases A Problem
Mar 17, 2025
-
Evidence Of Light As A Particle
Mar 17, 2025
-
Do Achiral Molecules Have A Plane Of Symmetry
Mar 17, 2025
-
Acto 3 Escena 1 Romeo Y Julieta
Mar 17, 2025
-
Can You Be In Love With Two People
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How To Make A Catalytic Triad . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
