Do Achiral Molecules Have A Plane Of Symmetry
Muz Play
Mar 17, 2025 · 5 min read
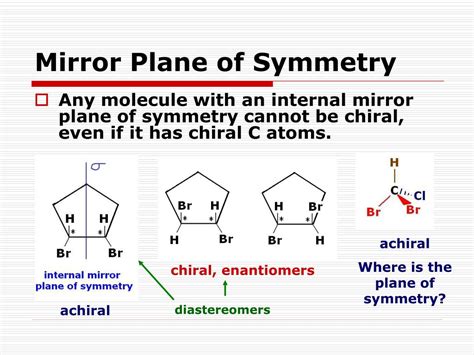
Table of Contents
Do Achiral Molecules Have a Plane of Symmetry? Understanding Chirality and Molecular Symmetry
Chirality, a fundamental concept in chemistry, dictates the three-dimensional arrangement of atoms in a molecule. Understanding chirality is crucial in various fields, including pharmaceuticals, materials science, and biochemistry, because it profoundly affects a molecule's properties and its interactions with other chiral molecules. A key aspect of determining whether a molecule is chiral or achiral lies in the presence or absence of a plane of symmetry. This article will delve deep into this relationship, explaining what constitutes a plane of symmetry and how it relates to the chirality of molecules.
What is Chirality?
Chirality describes the property of a molecule that cannot be superimposed on its mirror image. Imagine holding your left hand up to a mirror; the reflection is identical to your right hand. You can't overlay your left hand perfectly onto your right hand, despite their apparent similarity. This non-superimposability is the essence of chirality. Molecules exhibiting this property are called chiral, while those that are superimposable on their mirror images are achiral.
The Importance of Chirality
The implications of chirality are far-reaching:
-
Pharmacology: Many drugs exist as chiral molecules, and often, only one enantiomer (a specific type of chiral molecule) is responsible for the desired therapeutic effect. The other enantiomer might be inactive or even harmful. This is why pharmaceutical companies must carefully control the enantiomeric purity of their drugs. Thalidomide, a tragic example, highlights the devastating consequences of using a mixture of enantiomers without understanding their distinct effects.
-
Biochemistry: Living organisms are inherently chiral. Amino acids, the building blocks of proteins, are almost exclusively L-amino acids. Similarly, sugars in DNA and RNA are primarily D-sugars. This chirality profoundly influences the way biological molecules interact and function.
-
Materials Science: The chirality of molecules can affect the properties of materials, such as their crystallinity, optical activity, and mechanical strength. Chiral materials are increasingly being used in areas like liquid crystals and sensors.
Understanding Planes of Symmetry
A plane of symmetry, also known as a mirror plane, is an imaginary plane that bisects a molecule, dividing it into two halves that are mirror images of each other. If you could fold the molecule along this plane, the two halves would perfectly overlap. The presence or absence of such a plane is a critical criterion for determining chirality.
Identifying Planes of Symmetry
Identifying planes of symmetry requires careful visualization of the molecule's three-dimensional structure. Consider the following points:
-
Rotation: Rotating the molecule can reveal hidden planes of symmetry.
-
Perspective: Different perspectives can make identifying planes of symmetry easier.
-
Simple Molecules: In simpler molecules, planes of symmetry are often readily apparent.
-
Complex Molecules: For complex molecules, molecular modeling software can be invaluable in visualizing and identifying planes of symmetry.
The Relationship Between Achiral Molecules and Planes of Symmetry
The crucial point is this: all achiral molecules possess at least one plane of symmetry. The presence of this plane allows the molecule to be superimposed on its mirror image. Conversely, chiral molecules lack any plane of symmetry. This absence of symmetry is what makes them non-superimposable on their mirror images.
Examples of Achiral Molecules with Planes of Symmetry
Let's consider a few examples:
-
Methane (CH₄): Methane has several planes of symmetry. Any plane passing through the carbon atom and bisecting two opposing hydrogen atoms forms a plane of symmetry.
-
Dichloromethane (CH₂Cl₂): Dichloromethane also possesses planes of symmetry. One plane passes through the carbon atom and bisects the two chlorine atoms, while another plane passes through the carbon atom and bisects the two hydrogen atoms.
-
Benzene (C₆H₆): Benzene has multiple planes of symmetry, including planes passing through opposite carbon atoms and planes perpendicular to the ring.
Molecules Without Planes of Symmetry (Chiral Molecules)
Chiral molecules, by definition, lack any plane of symmetry. These molecules typically contain one or more stereocenters, which are typically carbon atoms bonded to four different substituents. A common example is a carbon atom bonded to four different alkyl groups or a combination of alkyl groups and other functional groups like halogens or hydroxyl groups.
-
Lactic Acid: Lactic acid is a chiral molecule commonly found in sour milk and muscles. It lacks a plane of symmetry due to its stereocenter.
-
2-Butanol: 2-Butanol possesses a stereocenter, making it a chiral molecule without a plane of symmetry.
-
Alanine: Alanine, an essential amino acid, is chiral and has no plane of symmetry.
Beyond Planes of Symmetry: Centers of Symmetry and Inversion Centers
While the presence or absence of a plane of symmetry is the most common way to determine chirality, it's important to note that other symmetry elements can also contribute. These include:
-
Center of Symmetry (Inversion Center): A point within the molecule through which all atoms can be reflected to find their mirror image on the opposite side. Molecules with a center of symmetry are achiral.
-
Rotation-Inversion Axis (Sₙ Axis): A combination of rotation and reflection operations. Molecules possessing an Sₙ axis (where n is an integer) are also achiral.
Practical Applications: Identifying Planes of Symmetry in Complex Molecules
Identifying planes of symmetry can be challenging, especially in larger, more complex molecules. Advanced techniques such as:
-
Molecular Modeling Software: Programs like Avogadro, ChemDraw, or GaussView allow for 3D visualization and rotation of molecules, simplifying the identification of planes of symmetry.
-
Symmetry Analysis: Computational methods can be employed to analyze the symmetry properties of a molecule automatically. These techniques are essential for studying complex structures where visual inspection is insufficient.
Conclusion: A Foundation for Understanding Molecular Properties
The presence or absence of a plane of symmetry is a fundamental aspect of molecular geometry with profound consequences for the physical and chemical properties of molecules. Achiral molecules, characterized by at least one plane of symmetry, are superimposable on their mirror images, while chiral molecules lack such symmetry, leading to unique properties and interactions. Understanding this distinction is paramount in various scientific disciplines, from drug design to materials science and biochemistry. The ability to identify planes of symmetry, particularly in complex molecules, remains a crucial skill for chemists and researchers working in these areas. Continued advancements in computational chemistry are instrumental in analyzing the symmetry of increasingly intricate molecular structures, further enriching our understanding of the fascinating world of chirality.
Latest Posts
Latest Posts
-
Find The Roots Of A Complex Number
Mar 17, 2025
-
After An Enzyme Reaction Is Completed The Enzyme
Mar 17, 2025
-
List The Functions Of Proteins In The Text Area Below
Mar 17, 2025
-
Subatomic Particle With A Negative Charge
Mar 17, 2025
-
Explain The Nature Of Colligative Properties
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Do Achiral Molecules Have A Plane Of Symmetry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
