Explain The Nature Of Colligative Properties
Muz Play
Mar 17, 2025 · 6 min read
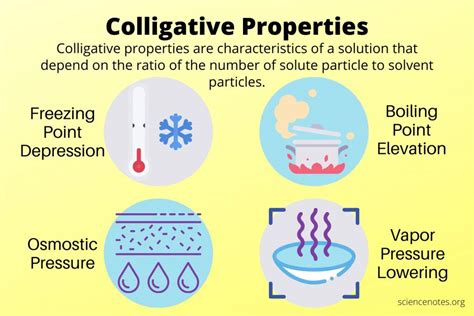
Table of Contents
Understanding the Nature of Colligative Properties
Colligative properties are a fascinating aspect of physical chemistry, describing how the physical properties of a solution differ from those of the pure solvent. These differences aren't due to the specific nature of the solute particles, but rather their number. In essence, colligative properties depend solely on the concentration of solute particles (molecules or ions) and not on their identity. This seemingly simple concept has profound implications across various fields, from everyday life to advanced chemical engineering. This article delves deep into the nature of colligative properties, exploring their underlying principles, individual characteristics, and diverse applications.
What are Colligative Properties?
Colligative properties are those properties of a solution that depend on the ratio of the number of solute particles to the number of solvent molecules in a solution, and not on the nature of the chemical species present. They are essentially a reflection of the solution's concentration, expressed in terms of molality (moles of solute per kilogram of solvent) or molarity (moles of solute per liter of solution), but molarity is less accurate as it's affected by temperature. The four main colligative properties are:
- Vapor Pressure Lowering: The reduction in vapor pressure of a solvent upon the addition of a non-volatile solute.
- Boiling Point Elevation: The increase in boiling point of a solvent when a non-volatile solute is added.
- Freezing Point Depression: The decrease in freezing point of a solvent when a non-volatile solute is added.
- Osmotic Pressure: The pressure required to prevent the flow of solvent across a semipermeable membrane from a region of low solute concentration to a region of high solute concentration.
The Underlying Principle: Raoult's Law and Ideal Solutions
The fundamental principle governing colligative properties is Raoult's Law, which states that the partial vapor pressure of each component in an ideal solution is equal to the product of the vapor pressure of the pure component and its mole fraction in the solution. This law holds true for ideal solutions, which are characterized by the following assumptions:
- No significant intermolecular interactions: The interactions between solute and solvent molecules are similar in strength to the interactions between molecules of the same type.
- Constant volume upon mixing: The total volume of the solution is equal to the sum of the volumes of the individual components.
- Molecules obey the ideal gas laws: Solute particles behave ideally, without significant interparticle forces.
While many solutions deviate from ideality, Raoult's Law serves as a valuable approximation, especially for dilute solutions where the solute concentration is low. Deviations from ideality are often observed in concentrated solutions or when there are strong interactions between solute and solvent molecules.
Detailed Explanation of Each Colligative Property
Let's examine each colligative property in detail, exploring its underlying mechanism and practical applications.
1. Vapor Pressure Lowering
Adding a non-volatile solute to a volatile solvent reduces the solvent's vapor pressure. This happens because the solute particles occupy some of the surface area of the solution, leaving fewer solvent molecules available to escape into the gas phase. The extent of vapor pressure lowering is directly proportional to the mole fraction of the solute. This relationship is mathematically expressed as:
P<sub>solution</sub> = X<sub>solvent</sub> * P<sup>o</sup><sub>solvent</sub>
where:
- P<sub>solution</sub> is the vapor pressure of the solution
- X<sub>solvent</sub> is the mole fraction of the solvent
- P<sup>o</sup><sub>solvent</sub> is the vapor pressure of the pure solvent
2. Boiling Point Elevation
The boiling point of a liquid is the temperature at which its vapor pressure equals the atmospheric pressure. Since adding a non-volatile solute lowers the vapor pressure, a higher temperature is required to reach the atmospheric pressure and achieve boiling. The elevation in boiling point (ΔT<sub>b</sub>) is directly proportional to the molality (m) of the solute:
ΔT<sub>b</sub> = K<sub>b</sub> * m
where:
- K<sub>b</sub> is the molal boiling point elevation constant, a characteristic property of the solvent.
3. Freezing Point Depression
Similarly, adding a solute lowers the freezing point of a solvent. The freezing point is the temperature at which the solid and liquid phases are in equilibrium. The presence of solute particles interferes with the formation of the solvent's crystalline structure, requiring a lower temperature to achieve this equilibrium. The depression in freezing point (ΔT<sub>f</sub>) is also directly proportional to the molality of the solute:
ΔT<sub>f</sub> = K<sub>f</sub> * m
where:
- K<sub>f</sub> is the molal freezing point depression constant, a characteristic property of the solvent.
4. Osmotic Pressure
Osmosis is the spontaneous movement of solvent molecules across a semipermeable membrane from a region of lower solute concentration to a region of higher solute concentration. Osmotic pressure (π) is the pressure required to stop this flow. It's directly proportional to the molar concentration (c) of the solute, the absolute temperature (T), and the ideal gas constant (R):
π = cRT
This equation is remarkably similar to the ideal gas law, highlighting the analogy between the behavior of solute particles in solution and gas molecules.
Applications of Colligative Properties
Colligative properties have wide-ranging applications in various fields:
- Determining Molar Mass: By measuring the freezing point depression or boiling point elevation of a solution, the molar mass of an unknown solute can be determined. This is a valuable technique in analytical chemistry.
- De-icing Agents: The freezing point depression of water by adding salts like NaCl is used extensively in de-icing roads and walkways during winter.
- Seawater Desalination: Osmosis plays a crucial role in reverse osmosis, a process used to desalinate seawater by applying pressure to force water through a semipermeable membrane, leaving the salts behind.
- Biological Systems: Osmotic pressure is essential in biological systems, maintaining the proper balance of water and solutes within cells.
- Automotive Coolants: Coolants used in automobiles typically contain antifreeze (ethylene glycol) to lower the freezing point and prevent engine damage in cold weather.
- Food Preservation: The addition of solutes like sugar or salt to food lowers the water activity, inhibiting microbial growth and extending shelf life.
Deviations from Ideality
It's crucial to acknowledge that many real-world solutions deviate from the ideal behavior predicted by Raoult's Law. These deviations arise primarily from significant interactions between solute and solvent molecules. For example, strong solute-solvent attractions can lead to a decrease in vapor pressure, while strong solute-solute interactions might cause an increase. These deviations are often more pronounced in concentrated solutions.
To account for these deviations, activity coefficients are introduced. Activity coefficients represent the effective concentration of a component in a non-ideal solution, correcting for the deviations from ideal behavior. The activity of a component is given by the product of its mole fraction and its activity coefficient. Using activities instead of mole fractions provides a more accurate representation of colligative properties in non-ideal solutions.
Conclusion
Colligative properties provide a powerful framework for understanding the behavior of solutions. While the ideal solution model provides a useful approximation, understanding deviations from ideality is vital for accurate predictions and applications. The ability to predict and control these properties has profound implications in diverse fields, demonstrating the broad relevance and continuing importance of this fundamental concept in physical chemistry. Further research in this area is always ongoing, focusing on improving models to handle non-ideal systems and developing new applications for these important properties. This deep understanding of colligative properties is fundamental to advancements in various fields, shaping innovations and solutions in chemistry, biology, and engineering.
Latest Posts
Latest Posts
-
The Ideal Osmotic Environment For An Animal Cell Is
Mar 17, 2025
-
What Are The Three Basic Components Of An Atom
Mar 17, 2025
-
Does Gas Have A Definite Shape
Mar 17, 2025
-
If The Equilibrium Constant Is Negative What Does That Mean
Mar 17, 2025
-
How Does An Atom Become A Cation
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Explain The Nature Of Colligative Properties . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
