The Ideal Osmotic Environment For An Animal Cell Is
Muz Play
Mar 17, 2025 · 6 min read
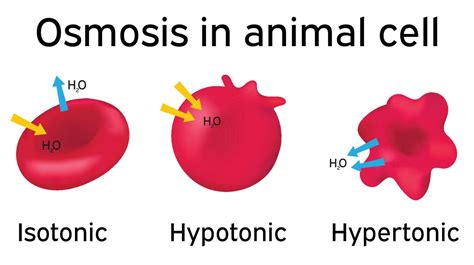
Table of Contents
The Ideal Osmotic Environment for an Animal Cell: Isotonic Bliss
Animal cells, the fundamental building blocks of animal life, are incredibly sensitive to their surrounding environment. Understanding their interaction with the surrounding solution, specifically its osmotic properties, is crucial to understanding cell function, health, and survival. This article delves deep into the intricacies of osmosis and its profound impact on animal cells, focusing on the ideal osmotic environment: isotonicity.
What is Osmosis?
Osmosis is the passive movement of water molecules across a selectively permeable membrane from a region of high water concentration to a region of low water concentration. This movement continues until equilibrium is reached, meaning the water concentration is equal on both sides of the membrane. The driving force behind osmosis is the difference in water potential, influenced by factors like solute concentration and pressure. Think of it like this: water wants to even out the concentration of solutes on either side of the membrane.
Selectively Permeable Membranes: The Gatekeepers
The selectively permeable membrane, primarily the cell membrane in animal cells, plays a critical role in osmosis. This membrane allows the passage of water molecules but restricts the movement of many solutes, ensuring controlled movement of substances into and out of the cell. This selectivity is vital for maintaining the cell's internal environment.
Osmotic Environments: A Triad of Conditions
Animal cells can experience three main osmotic environments:
1. Isotonic Solution: The Goldilocks Zone
An isotonic solution is one where the concentration of solutes outside the cell is equal to the concentration of solutes inside the cell. In simpler terms, the water potential inside and outside the cell is the same. This is the ideal osmotic environment for most animal cells. In an isotonic solution, there's no net movement of water across the cell membrane. Water molecules move across the membrane in both directions at equal rates, maintaining a stable cell volume and preventing cell damage. This equilibrium is essential for normal cell function and prevents the cell from either shrinking or bursting.
Keywords: Isotonic solution, ideal osmotic environment, equilibrium, cell volume, cell function, normal cell function, osmotic balance.
Maintaining Isotonicity: A Delicate Balance
Maintaining isotonicity is a crucial aspect of homeostasis, the process by which organisms maintain a stable internal environment. Various mechanisms, including active transport and ion channels, play a role in regulating the movement of solutes and maintaining the isotonic state. Any significant deviation from isotonicity can have serious consequences for cell health.
2. Hypotonic Solution: Swelling and Potential Rupture
A hypotonic solution has a lower solute concentration than the inside of the cell. This means there is a higher concentration of water outside the cell compared to inside. Because water moves from an area of high concentration to an area of low concentration, water rushes into the cell via osmosis. The cell swells as it takes in water. If the difference in concentration is significant, the cell may swell to the point of bursting, a process called lysis. Animal cells, lacking a rigid cell wall like plant cells, are particularly vulnerable to lysis in hypotonic solutions.
Keywords: Hypotonic solution, lysis, cell swelling, water influx, osmotic pressure, cell damage, homeostasis disruption.
Examples of Hypotonic Environments
Exposure to pure water is a classic example of a hypotonic environment for an animal cell. The extremely low solute concentration outside the cell causes rapid water influx and potential cell lysis. Many freshwater organisms have evolved specialized mechanisms to deal with the hypotonic environment of their surroundings.
3. Hypertonic Solution: Shrinkage and Cellular Distress
A hypertonic solution has a higher solute concentration than the inside of the cell. This means the water concentration is lower outside the cell. Consequently, water moves out of the cell via osmosis, causing the cell to shrink. This process is called crenation or plasmolysis. As the cell loses water, its cytoplasm shrinks, and the cell membrane pulls away from the cell wall (if present). This shrinkage can disrupt cellular processes and lead to cell death.
Keywords: Hypertonic solution, crenation, plasmolysis, water efflux, cellular dehydration, cell shrinkage, impaired cell function, osmotic stress.
Examples of Hypertonic Environments
High salt concentrations, such as those found in seawater, create hypertonic environments for many animal cells. Marine animals have developed various adaptations, such as specialized osmoregulatory organs, to cope with the hypertonic conditions of their habitat.
The Importance of Maintaining Isotonicity
Maintaining an isotonic environment is paramount for the health and proper functioning of animal cells. Here's why:
-
Cellular Integrity: Isotonicity ensures that the cell maintains its structural integrity. Neither swelling nor shrinkage occurs, preventing damage to cellular components.
-
Optimal Metabolic Function: Proper cell volume is essential for the efficient functioning of cellular processes, including metabolism, transport, and signaling.
-
Preventing Cell Death: Exposure to hypotonic or hypertonic environments can lead to cell death, either through lysis or crenation. Maintaining isotonicity prevents this premature cell death.
-
Homeostasis: Isotonicity plays a vital role in maintaining cellular homeostasis, which is crucial for overall organismal health.
Osmosis and Osmoregulation: A Dynamic Duo
Many animals have developed complex osmoregulatory systems to maintain isotonicity in their cells, despite variations in the osmotic environment of their surroundings. These systems actively regulate the concentration of solutes and water in body fluids, ensuring that cells remain in an isotonic state.
Examples of Osmoregulation:
-
Kidneys: The kidneys play a critical role in osmoregulation, filtering blood and removing excess water and waste products. They also regulate the concentration of electrolytes, contributing to the maintenance of isotonicity.
-
Gills in Fish: Fish gills are involved in osmoregulation, enabling them to control the movement of water and ions across their body surfaces. Freshwater fish face a hypotonic environment, while saltwater fish face a hypertonic environment. Their gills enable them to actively maintain osmotic balance.
-
Specialized Cells: Some cells have specialized mechanisms, like contractile vacuoles in some single-celled organisms, to pump out excess water from the cytoplasm, preventing lysis in hypotonic environments.
Clinical Implications: IV Fluids and Isotonicity
Understanding osmosis is critical in clinical settings, particularly in fluid therapy. Intravenous (IV) fluids must be isotonic to prevent damage to red blood cells and other cells in the bloodstream. Using hypotonic or hypertonic solutions can have serious consequences, leading to hemolysis (rupture of red blood cells) or crenation, respectively. The careful selection of isotonic fluids is crucial for maintaining the patient's fluid balance and preventing complications.
Conclusion: Isotonicity - A Cellular Necessity
The ideal osmotic environment for an animal cell is an isotonic solution. Maintaining this isotonic state is crucial for preserving cellular integrity, ensuring optimal metabolic function, preventing cell death, and contributing to overall organismal homeostasis. The complexities of osmosis and the diverse adaptations of animals to maintain osmotic balance underscore the vital role of this process in life. From the microscopic level of individual cells to the macroscopic level of entire organisms, the quest for isotonicity is a constant biological imperative. Further research into this field continues to expand our understanding of cell biology and the intricate mechanisms that maintain the delicate balance of life.
Latest Posts
Latest Posts
-
Are Anions Bigger Than Neutral Atoms
Mar 17, 2025
-
Limiting Reactant And Percent Yield Lab
Mar 17, 2025
-
What Are The Four Agents Of Socialization
Mar 17, 2025
-
What Do Humans Need To Survive
Mar 17, 2025
-
How Many Covalent Bonds Does Oxygen Have
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about The Ideal Osmotic Environment For An Animal Cell Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
