Are Anions Bigger Than Neutral Atoms
Muz Play
Mar 17, 2025 · 5 min read
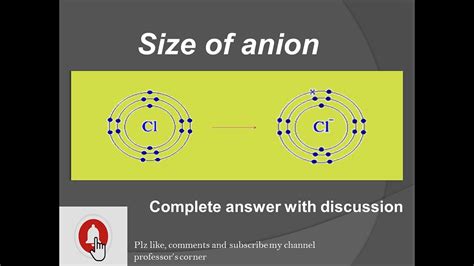
Table of Contents
Are Anions Bigger Than Neutral Atoms? A Deep Dive into Ionic Radii
Understanding the size of atoms and ions is crucial in chemistry, impacting properties like reactivity, bonding, and crystal structure. A common question that arises is whether anions (negatively charged ions) are larger than their corresponding neutral atoms. The answer, unequivocally, is yes. This article will delve into the reasons behind this phenomenon, exploring the underlying principles of electron configuration and electrostatic interactions. We'll also examine exceptions and nuances to this general rule.
The Role of Electron-Electron Repulsion
The fundamental reason why anions are larger than neutral atoms lies in the increased electron-electron repulsion. When an atom gains an electron to become an anion, it adds to its existing electron cloud. This addition doesn't simply occupy an empty space; it increases the overall negative charge and the number of electrons vying for the same space around the nucleus.
Shielding Effect and Effective Nuclear Charge
The nucleus, positively charged, attracts electrons. However, the electrons themselves repel each other. This repulsion is termed the shielding effect. The inner electrons shield the outer electrons from the full positive charge of the nucleus. This shielding reduces the effective nuclear charge experienced by the outer electrons.
When an atom gains an electron to form an anion, the number of electrons increases, increasing the shielding effect. This further reduces the effective nuclear charge felt by each electron. Consequently, the electrons are less tightly held by the nucleus, leading to an expansion of the electron cloud and a larger ionic radius.
In simpler terms: Imagine a group of people (electrons) around a campfire (nucleus). Adding more people to the group (adding electrons) increases the pushing and shoving (electron-electron repulsion), making the group spread out more.
Comparing Ionic and Atomic Radii: Examples
Let's consider some specific examples to illustrate the size difference between anions and their neutral atoms:
-
Oxygen (O) and Oxide (O²⁻): A neutral oxygen atom has eight electrons. When it gains two electrons to form the oxide anion (O²⁻), it now has ten electrons. The increased electron-electron repulsion causes the electron cloud to expand significantly, resulting in a much larger ionic radius for O²⁻ compared to O.
-
Chlorine (Cl) and Chloride (Cl⁻): Chlorine, with 17 electrons, readily gains one electron to form the chloride anion (Cl⁻). This added electron increases the electron-electron repulsion, expanding the electron cloud and making Cl⁻ larger than Cl.
-
Fluorine (F) and Fluoride (F⁻): Similar to chlorine, fluorine readily gains an electron to become fluoride (F⁻). The same principle applies: increased electron-electron repulsion leads to a larger ionic radius for the anion.
These examples consistently demonstrate the trend: anions are larger than their corresponding neutral atoms.
Factors Influencing Anion Size
While increased electron-electron repulsion is the primary driver, other factors subtly influence the size of anions:
-
Nuclear Charge: A higher nuclear charge attracts electrons more strongly, counteracting the expansion caused by added electrons. However, the increase in electron-electron repulsion generally outweighs the effect of a higher nuclear charge in most cases.
-
Electron Shell: Adding electrons to a new principal energy level (shell) causes a significant increase in size. This effect is more pronounced than the gradual expansion within the same shell.
-
Electron Configuration: The specific electron configuration affects the extent of electron-electron repulsion. Electrons in the same subshell repel each other more strongly than those in different subshells.
Exceptions and Nuances
While the general rule holds true, there are subtle exceptions and nuances:
-
Transition Metal Ions: Transition metal ions often show less predictable trends in ionic radii due to the complex interplay of factors like electron configuration and shielding effects. The involvement of d- and f-orbitals introduces complexities that deviate from the simple model described above.
-
Highly Charged Anions: For highly charged anions, the increased nuclear charge might exert a more significant influence, somewhat mitigating the effect of electron-electron repulsion. However, the size increase is still typically observed.
Applications and Importance
Understanding the relative sizes of anions and neutral atoms has numerous applications in various fields:
-
Crystallography: The sizes of ions are crucial in determining the crystal structure of ionic compounds. The arrangement of ions in a crystal lattice is directly related to their relative sizes and the balance of electrostatic attractions and repulsions.
-
Solubility: The size of ions influences their solubility in different solvents. Larger ions often have weaker interactions with solvent molecules, potentially affecting their solubility.
-
Reactivity: The size of an anion impacts its reactivity. Larger anions generally have a more diffuse electron cloud, making them less reactive than smaller anions.
Conclusion: A Consistent Trend
In summary, anions are generally larger than their corresponding neutral atoms. This difference in size is primarily due to the increase in electron-electron repulsion when an atom gains electrons. While several factors can subtly influence the precise size difference, the fundamental principle remains consistent: the addition of electrons to a neutral atom leads to an expansion of the electron cloud and a larger ionic radius. Understanding this principle is vital for comprehending the behavior and properties of ions in various chemical and physical systems. Further research and advanced techniques continue to refine our understanding of ionic radii and their implications across diverse scientific disciplines. The exploration of ionic sizes remains a dynamic area of study, with ongoing advancements enhancing our knowledge of atomic and molecular structures.
Latest Posts
Latest Posts
-
Which Statement Summarizes The Law Of Segregation
Mar 18, 2025
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
-
Is Salt Water A Pure Substance
Mar 18, 2025
-
What Are The Functions Of The Family
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Are Anions Bigger Than Neutral Atoms . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
