Is Salt Water A Pure Substance
Muz Play
Mar 18, 2025 · 6 min read
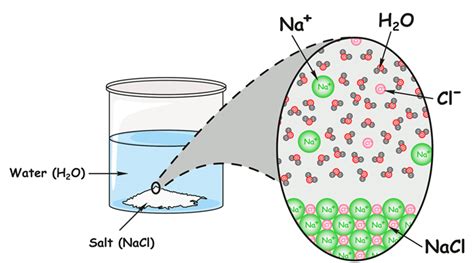
Table of Contents
Is Salt Water a Pure Substance? Delving into Mixtures and Solutions
The question, "Is saltwater a pure substance?" seems simple, but it opens a fascinating door into the world of chemistry and the precise definitions of matter. The short answer is no, saltwater is not a pure substance. Understanding why requires exploring the concepts of pure substances, mixtures, and solutions. This article will delve deep into these concepts, explaining why saltwater is classified as a mixture and exploring the properties that distinguish it from pure substances. We’ll also touch upon the implications of this classification in various scientific fields.
Understanding Pure Substances
A pure substance is defined as a form of matter that has a constant composition and properties throughout the sample. This means that regardless of where you take a sample from a given pure substance, its chemical makeup will be identical. Pure substances can be further categorized into two types:
Elements
Elements are the fundamental building blocks of matter. They are substances that cannot be broken down into simpler substances by chemical means. The periodic table organizes all known elements. Examples include oxygen (O), iron (Fe), gold (Au), and hydrogen (H). Each element has unique physical and chemical properties.
Compounds
Compounds are pure substances formed when two or more elements chemically combine in fixed proportions. These elements are bound together by chemical bonds, resulting in a new substance with properties different from the constituent elements. Examples include water (H₂O), where two hydrogen atoms bond with one oxygen atom, and table salt (NaCl), where one sodium atom bonds with one chlorine atom. The ratio of elements in a compound is always constant. You can't have a compound that's sometimes H₂O and sometimes H₃O, for instance.
Exploring Mixtures
In contrast to pure substances, mixtures are combinations of two or more substances that are not chemically bonded. The components retain their individual properties, and their proportions can vary. Mixtures can be further classified into two main types:
Homogeneous Mixtures
Homogeneous mixtures have a uniform composition throughout. This means that the different components are evenly distributed at a macroscopic level, and you can't easily distinguish them visually. Saltwater is a prime example of a homogeneous mixture. When salt (NaCl) dissolves in water (H₂O), the sodium and chloride ions become evenly dispersed throughout the water molecules. You can't see individual salt crystals, and the solution appears clear and uniform. Other examples include air (a mixture of gases) and many alloys (mixtures of metals).
Heterogeneous Mixtures
Heterogeneous mixtures have a non-uniform composition. You can visually distinguish the different components. Think of a salad, where you can see the distinct lettuce, tomatoes, and cucumbers. Or consider sand and water; the sand particles are clearly separated from the water. The proportions of the components can also vary in different parts of the mixture.
Saltwater: A Detailed Look at a Homogeneous Mixture
Now, let's return to saltwater. As mentioned, it's a homogeneous mixture. It's a solution where sodium chloride (NaCl) is the solute, and water (H₂O) is the solvent. The salt dissolves in the water, forming a solution where the ions are dispersed evenly.
The Dissolution Process
The process of dissolving salt in water involves several steps. The polar water molecules, with their slightly positive and negative ends, interact with the ions of the salt crystal. The positive end of the water molecule attracts the negatively charged chloride ions (Cl⁻), while the negative end attracts the positively charged sodium ions (Na⁺). These interactions weaken the ionic bonds holding the salt crystal together, and the ions become surrounded by water molecules, a process called hydration. The hydrated ions are then free to move throughout the solution.
Properties of Saltwater
The properties of saltwater are different from those of pure water and pure salt. For example:
- Boiling point: Saltwater has a higher boiling point than pure water. The dissolved salt ions interfere with the water molecules' ability to escape into the gaseous phase, requiring more energy (higher temperature) to reach the boiling point.
- Freezing point: Saltwater has a lower freezing point than pure water. The dissolved ions disrupt the formation of the crystal lattice structure of ice, making it harder for the water to freeze.
- Density: Saltwater is denser than pure water. The added mass of the salt ions increases the overall density of the solution.
- Electrical conductivity: Saltwater is an electrical conductor, unlike pure water. The dissolved ions can carry an electric current.
These altered properties demonstrate that saltwater is not a simple combination of water and salt; the interactions between the components create a new set of characteristics.
Distinguishing between Mixtures and Compounds
It's crucial to understand the fundamental difference between mixtures and compounds. While both involve multiple substances, the key lies in the chemical bonding:
- Mixtures: Substances are physically combined, retaining their individual chemical properties. They can be separated by physical methods like filtration, distillation, or evaporation.
- Compounds: Substances are chemically combined, forming new substances with different properties. They can only be separated by chemical reactions.
Saltwater can be separated into its components—salt and water—through physical methods like evaporation. The salt is left behind as the water evaporates, demonstrating its nature as a mixture. This contrasts with a compound like water (H₂O), which cannot be separated into hydrogen and oxygen through simple physical means; chemical processes are required.
Implications in Different Fields
The distinction between pure substances and mixtures has significant implications in various scientific and technological fields:
- Chemistry: Understanding the nature of mixtures and solutions is fundamental to stoichiometry, solution chemistry, and many other areas. The properties of solutions, like saltwater, differ significantly from those of their components.
- Oceanography: Ocean water is a complex mixture, and its composition plays a crucial role in marine ecosystems and ocean currents. The salinity of seawater significantly impacts its density and temperature, influencing its movement and the organisms that inhabit it.
- Medicine: Intravenous (IV) solutions are meticulously prepared mixtures designed to deliver specific substances to the bloodstream. The precise composition is crucial for patient health.
- Environmental Science: Water quality analysis often focuses on identifying the various substances present in water samples, differentiating between pure water and mixtures containing pollutants.
- Food Science: Many food products are mixtures, and their composition impacts their properties, shelf life, and nutritional value.
Conclusion: Saltwater – A Complex Mixture
In conclusion, saltwater is unequivocally not a pure substance. It's a homogeneous mixture, specifically a solution, where salt dissolves in water. The components retain their individual identities but interact to create a new set of properties. Understanding this distinction between pure substances and mixtures is fundamental across various scientific disciplines and technological applications. The properties and behavior of saltwater are a testament to the rich complexity of mixtures and their importance in the natural world and human endeavors. The seemingly simple question of whether saltwater is a pure substance has led us on a journey exploring the fundamental concepts of matter and the fascinating interactions between its various forms.
Latest Posts
Latest Posts
-
What Is The Total Magnification Of 4x
Mar 18, 2025
-
The Cutaneous Membrane Is Blank To The Muscles
Mar 18, 2025
-
If A Hybrid Offspring Does Not Survive
Mar 18, 2025
-
5 Postulates Of Daltons Atomic Theory
Mar 18, 2025
-
How To Find Linear Relationship Between Independent And Dependent Variables
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is Salt Water A Pure Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
