Limiting Reactant And Percent Yield Lab
Muz Play
Mar 17, 2025 · 6 min read
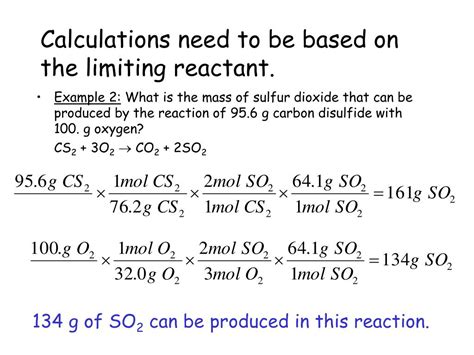
Table of Contents
Limiting Reactant and Percent Yield Lab: A Comprehensive Guide
The concepts of limiting reactants and percent yield are fundamental in chemistry, forming the bedrock of stoichiometric calculations and real-world applications. This lab explores these concepts through hands-on experimentation, solidifying understanding and highlighting the practical limitations of theoretical calculations. This detailed guide will walk you through the process, from pre-lab preparations to post-lab analysis, ensuring you gain a comprehensive grasp of these crucial chemical principles.
Understanding Limiting Reactants
In any chemical reaction involving multiple reactants, one reactant will inevitably be consumed completely before the others. This reactant is known as the limiting reactant because it limits the amount of product that can be formed. The other reactants, present in excess, are called excess reactants. Identifying the limiting reactant is crucial for predicting the maximum amount of product that can be produced, a quantity known as the theoretical yield.
Identifying the Limiting Reactant: A Step-by-Step Approach
-
Balanced Chemical Equation: Begin with a correctly balanced chemical equation. This ensures the correct mole ratios between reactants and products.
-
Moles of Reactants: Convert the given masses of each reactant into moles using their respective molar masses.
-
Mole Ratio Comparison: Use the stoichiometric coefficients from the balanced equation to determine the mole ratio between the reactants. Compare the actual mole ratio to the stoichiometric mole ratio.
-
Limiting Reactant Determination: The reactant with the smaller mole ratio (compared to the stoichiometric ratio) is the limiting reactant. This reactant will be completely consumed first, thereby limiting the reaction's progress.
Understanding Percent Yield
The theoretical yield represents the maximum possible amount of product that can be formed based on stoichiometric calculations, assuming 100% reaction efficiency. However, in reality, chemical reactions rarely achieve 100% efficiency due to various factors such as incomplete reactions, side reactions, and experimental errors. This is where the concept of percent yield comes into play.
The percent yield expresses the ratio of the actual yield (the amount of product actually obtained) to the theoretical yield, multiplied by 100%:
Percent Yield = (Actual Yield / Theoretical Yield) x 100%
A high percent yield (close to 100%) indicates a highly efficient reaction, while a low percent yield suggests significant losses or inefficiencies.
Lab Procedure: Determining Limiting Reactant and Percent Yield
This lab will focus on a specific reaction (choose a suitable reaction based on available materials and safety considerations. Examples include the reaction between sodium bicarbonate and acetic acid to produce carbon dioxide, or a precipitation reaction like the reaction between lead(II) nitrate and potassium iodide). Remember to adapt the procedure and calculations based on the chosen reaction.
Materials:
- (Specify materials based on the chosen reaction, including reactants, glassware, measuring instruments, etc.)
Procedure:
-
Pre-lab Calculations: Before starting the experiment, calculate the theoretical yield based on the given amounts of reactants. Identify the limiting reactant through the steps described above. Record all calculations clearly.
-
Reaction Setup: Carefully measure and combine the reactants according to the balanced chemical equation and safety protocols. Ensure thorough mixing to maximize reaction efficiency.
-
Reaction Execution: Allow the reaction to proceed completely, monitoring its progress (e.g., observing gas evolution, precipitate formation, or temperature change). Record any observations.
-
Product Isolation and Purification: Once the reaction is complete, isolate and purify the product. The method depends on the nature of the product. For instance, if it's a precipitate, you'll need to filter and dry it. If it's a gas, you might collect it over water and measure its volume.
-
Product Mass Determination: Accurately measure the mass or volume of the purified product obtained (actual yield). Ensure that the product is completely dry before weighing.
-
Percent Yield Calculation: Using the actual yield and the previously calculated theoretical yield, compute the percent yield using the formula above.
Data Analysis and Interpretation
-
Record all observations meticulously, including qualitative aspects (e.g., color changes, precipitate formation, gas evolution) and quantitative data (e.g., mass of reactants, mass of product, volume of gas).
-
Calculate the theoretical yield for the reaction based on the limiting reactant. Show all your work, including unit conversions and mole ratios.
-
Determine the actual yield by accurately measuring the mass or volume of the isolated and purified product.
-
Calculate the percent yield using the formula: (Actual Yield / Theoretical Yield) x 100%.
-
Analyze sources of error. Identify factors that might have contributed to a percent yield less than 100%. Common sources of error include incomplete reactions, loss of product during isolation and purification, side reactions, and experimental errors in measurement.
-
Discuss potential improvements. Suggest ways to improve the experimental procedure to obtain a higher percent yield in future experiments. This could include using more precise measuring instruments, optimizing reaction conditions (temperature, pressure, concentration), or employing different purification techniques.
Advanced Considerations: Error Analysis and Uncertainty
No experiment is perfectly precise. Understanding and quantifying uncertainty is crucial for a robust scientific investigation.
-
Uncertainty in Measurements: Every measurement has inherent uncertainty associated with the instrument used. Consider the uncertainty in measuring masses, volumes, and temperatures. Properly propagate this uncertainty through your calculations to determine the uncertainty in the final percent yield.
-
Systematic Errors: These errors are consistent and repeatable, often stemming from flaws in the experimental setup or procedure. Identify potential systematic errors in your experiment and discuss their impact on the results.
-
Random Errors: These errors are unpredictable and fluctuate randomly. Multiple trials can help reduce the effect of random errors on the final results.
-
Statistical Analysis: For multiple trials, calculate the average percent yield and its standard deviation to better represent the accuracy and precision of the experiment.
Safety Precautions
Always prioritize safety when conducting chemical experiments. Wear appropriate safety goggles and gloves, work in a well-ventilated area, and handle chemicals responsibly according to the provided safety data sheets (SDS). Properly dispose of all waste materials according to laboratory safety protocols.
Conclusion
This lab provides a hands-on experience in understanding the crucial concepts of limiting reactants and percent yield. By carefully executing the procedure and thoroughly analyzing the results, you will gain a deeper appreciation for the practical limitations of stoichiometric calculations and the importance of accurate measurements and error analysis in chemical experiments. The knowledge gained will serve as a strong foundation for further exploration in chemical stoichiometry and reaction kinetics. Remember to adapt this procedure and calculations to the specific reaction you choose for your experiment. The key is to focus on understanding the underlying principles and applying them rigorously to your data.
Latest Posts
Latest Posts
-
Which One Leaves The Solution Untouched
Mar 18, 2025
-
Which Statement Summarizes The Law Of Segregation
Mar 18, 2025
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
-
Is Salt Water A Pure Substance
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Limiting Reactant And Percent Yield Lab . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
