What Are The Three Basic Components Of An Atom
Muz Play
Mar 17, 2025 · 6 min read
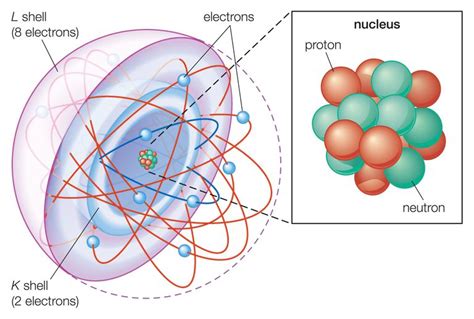
Table of Contents
What Are the Three Basic Components of an Atom? A Deep Dive into Atomic Structure
Understanding the atom is fundamental to understanding all of chemistry and physics. Everything around us, from the air we breathe to the ground beneath our feet, is made up of atoms. But what exactly is an atom? This article will delve into the three basic components of an atom: protons, neutrons, and electrons, exploring their properties, interactions, and the overall structure they create.
The Three Pillars of the Atom: Protons, Neutrons, and Electrons
Atoms are incredibly tiny, far too small to be seen with even the most powerful optical microscopes. Yet, these minuscule particles hold the key to the universe's complexity. At the heart of every atom lies the nucleus, a dense region containing two types of particles: protons and neutrons. Surrounding the nucleus is a cloud of electrons, whizzing around at incredible speeds.
1. Protons: The Positively Charged Core
Protons are subatomic particles carrying a single positive electrical charge. They are significantly more massive than electrons, contributing significantly to the atom's overall mass. The number of protons in an atom's nucleus defines its atomic number, which uniquely identifies the element. For instance, hydrogen has one proton (atomic number 1), helium has two (atomic number 2), and so on. This number is crucial because it determines the element's chemical properties and its place on the periodic table.
Key Properties of Protons:
- Positive charge: +1 elementary charge
- Mass: Approximately 1 atomic mass unit (amu)
- Location: Nucleus
- Symbol: p⁺ or p
2. Neutrons: The Neutral Stabilizers
Neutrons are subatomic particles with no electrical charge, hence their name. They are slightly more massive than protons, also contributing to the atom's mass. Neutrons play a vital role in nuclear stability. In many atoms, the number of neutrons is roughly equal to the number of protons. However, the ratio can vary, leading to isotopes – atoms of the same element with different numbers of neutrons. Some isotopes are stable, while others are radioactive, meaning they decay over time, emitting radiation.
Key Properties of Neutrons:
- Neutral charge: 0
- Mass: Approximately 1 atomic mass unit (amu)
- Location: Nucleus
- Symbol: n⁰
3. Electrons: The Orbiting Negatively Charged Particles
Electrons are subatomic particles carrying a single negative electrical charge. They are significantly lighter than protons and neutrons, with a mass approximately 1/1836th of a proton's mass. Electrons reside outside the nucleus in regions called electron shells or energy levels. These shells represent different energy states, with electrons in lower shells being closer to the nucleus and having lower energy.
The arrangement of electrons in these shells determines the atom's chemical behavior. Atoms tend to react with other atoms to achieve a stable electron configuration, often by filling their outermost shell (valence shell). This tendency drives chemical bonding, leading to the formation of molecules and compounds.
Key Properties of Electrons:
- Negative charge: -1 elementary charge
- Mass: Approximately 1/1836 amu
- Location: Electron shells surrounding the nucleus
- Symbol: e⁻
The Atomic Nucleus: A Powerful and Tiny Core
The nucleus is the atom's central region, containing both protons and neutrons. It's incredibly dense, accounting for almost all of the atom's mass, yet occupying only a tiny fraction of its volume. The strong nuclear force, one of the fundamental forces of nature, holds the protons and neutrons together within the nucleus. This force is much stronger than the electromagnetic force that repels the positively charged protons, preventing the nucleus from flying apart.
The size of the nucleus is incredibly small, on the order of femtometers (10⁻¹⁵ meters). To put this in perspective, if an atom were the size of a football stadium, the nucleus would be about the size of a pea in the center.
Nuclear Stability and Isotopes
The ratio of protons to neutrons in the nucleus is crucial for its stability. Atoms with a balanced ratio tend to be stable, while those with an imbalanced ratio may be radioactive. Radioactive isotopes undergo radioactive decay, emitting particles or energy to achieve a more stable configuration. This decay can be harnessed for various applications, including medical imaging and cancer treatment.
Electron Shells and Energy Levels: The Quantum Realm
Electrons don't orbit the nucleus in simple, predictable paths like planets around the sun. Instead, their behavior is governed by the principles of quantum mechanics. Electrons exist in regions of space called orbitals, which represent the probability of finding an electron at a particular location. These orbitals are grouped into shells or energy levels, each capable of holding a specific number of electrons.
The lowest energy level (closest to the nucleus) can hold up to two electrons. Subsequent energy levels can hold increasing numbers of electrons. The arrangement of electrons in these shells determines the atom's chemical reactivity and its properties. Atoms with filled outer shells are generally unreactive (inert), while those with partially filled outer shells are more reactive.
Valence Electrons and Chemical Bonding
The electrons in the outermost shell are called valence electrons. These electrons are the most involved in chemical reactions. Atoms tend to react with each other in ways that either fill their valence shells (resulting in stable electron configurations) or share valence electrons to achieve stability. This leads to the formation of chemical bonds, the forces that hold atoms together in molecules and compounds.
The Importance of Atomic Structure: From Matter to Technology
Understanding the three basic components of an atom – protons, neutrons, and electrons – is fundamental to comprehending the behavior of matter at its most fundamental level. This knowledge has profoundly impacted various fields, including:
- Chemistry: Atomic structure is the cornerstone of all chemical reactions and the formation of molecules and compounds.
- Physics: The study of atomic structure has led to breakthroughs in nuclear physics, particle physics, and quantum mechanics.
- Materials Science: Understanding atomic structure allows scientists to design and create new materials with specific properties.
- Medicine: Radioisotopes, derived from unstable isotopes, are used in medical imaging and cancer treatment.
- Technology: Advancements in electronics, computing, and energy technologies rely heavily on the understanding of atomic structure and its applications.
Conclusion: A Journey into the Subatomic World
The seemingly simple atom is a marvel of complexity and elegance. Its three basic components—protons, neutrons, and electrons—interact to create the rich diversity of matter that makes up our universe. Understanding the intricacies of atomic structure is not just an academic pursuit but a key to unlocking the potential of science and technology, paving the way for future innovations and discoveries. The ongoing exploration of the subatomic world continues to reveal new and fascinating aspects of the universe, highlighting the profound impact of understanding the building blocks of all matter.
Latest Posts
Latest Posts
-
Limiting Reactant And Percent Yield Lab
Mar 17, 2025
-
What Are The Four Agents Of Socialization
Mar 17, 2025
-
What Do Humans Need To Survive
Mar 17, 2025
-
How Many Covalent Bonds Does Oxygen Have
Mar 17, 2025
-
Identify The Equation For The Graph
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Are The Three Basic Components Of An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
