If The Equilibrium Constant Is Negative What Does That Mean
Muz Play
Mar 17, 2025 · 5 min read
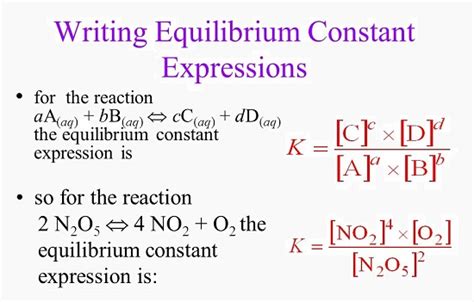
Table of Contents
If the Equilibrium Constant is Negative: What Does That Mean?
The equilibrium constant, denoted as K, is a fundamental concept in chemistry that quantifies the relative amounts of reactants and products present at equilibrium in a reversible reaction. Understanding the equilibrium constant is crucial for predicting the direction and extent of a reaction. A common misconception surrounds the possibility of a negative equilibrium constant. This article will delve into the meaning of the equilibrium constant, explore why it's always non-negative, and clarify the situations that might lead to confusion about its sign.
Understanding the Equilibrium Constant (K)
The equilibrium constant is a ratio of the activities (or concentrations, in simpler cases) of products to reactants at equilibrium, each raised to the power of its stoichiometric coefficient in the balanced chemical equation. For a general reversible reaction:
aA + bB ⇌ cC + dD
The equilibrium constant expression is:
K = ([C]^c[D]^d) / ([A]^a[B]^b)
where:
- [A], [B], [C], and [D] represent the equilibrium concentrations (or activities) of reactants and products.
- a, b, c, and d are the stoichiometric coefficients from the balanced chemical equation.
A large value of K (K >> 1) indicates that the equilibrium lies far to the right, meaning that the products are heavily favored at equilibrium. A small value of K (K << 1) indicates that the equilibrium lies far to the left, meaning that the reactants are heavily favored. A value of K close to 1 suggests that comparable amounts of reactants and products are present at equilibrium.
Crucially, the equilibrium constant is always a positive value, or technically, a non-negative value that can be zero. This stems directly from the mathematical definition – it is a ratio of concentrations (or activities) raised to positive powers. Concentrations are always non-negative. Therefore, even if the numerator or denominator is small (approaching zero), the ratio itself will always remain non-negative.
Why a Negative Equilibrium Constant is Impossible
The notion of a negative K arises from a misunderstanding of the mathematical definition and the physical reality of chemical equilibria. Several scenarios might lead to confusion about a negative equilibrium constant:
-
Incorrect Calculation: The most common reason for apparent negative K values is simply an error in the calculation. Double-check the balanced chemical equation, the equilibrium concentrations, and the application of the equilibrium constant expression. Ensure that you're using the correct stoichiometric coefficients and that the units are consistent.
-
Ignoring Activity Coefficients: In more advanced treatments of chemical equilibrium, activity coefficients are used to account for deviations from ideal behavior, especially in concentrated solutions. These coefficients correct for intermolecular interactions. Failing to incorporate activity coefficients can lead to inaccurate calculations and potentially confusing results. While activity coefficients can be less than 1, they are always positive, hence the K value remains positive.
-
Misinterpretation of Gibbs Free Energy: The equilibrium constant is related to the standard Gibbs free energy change (ΔG°) of the reaction through the following equation:
ΔG° = -RTlnK
where:
- R is the ideal gas constant
- T is the temperature in Kelvin.
A negative ΔG° indicates a spontaneous reaction under standard conditions (favoring products), and a positive ΔG° indicates a non-spontaneous reaction under standard conditions (favoring reactants). However, even if ΔG° is positive, K will still be positive, but a value less than 1. A negative ΔG° simply indicates a larger K value (K > 1). The sign of ΔG° does not translate directly to the sign of K.
Potential Sources of Confusion: Specific Reaction Types
Let's address some scenarios that might initially seem to suggest a negative equilibrium constant:
-
Reactions Involving Gases with Partial Pressures: When dealing with gaseous equilibria, partial pressures are often used instead of concentrations. However, partial pressures, like concentrations, are always positive values. The equilibrium constant expression then uses partial pressures instead of concentrations, but the principle remains the same: K will always be positive.
-
Complex Equilibria: In complex equilibria, such as those involving multiple steps or simultaneous reactions, the overall equilibrium constant might seem complex to calculate, but the individual equilibrium constants for each step remain positive.
-
Acid-Base Equilibria: In acid-base equilibria, the equilibrium constant is often expressed as Ka (acid dissociation constant) or Kb (base dissociation constant). While the numerical value of Ka or Kb can be less than one (indicating weak acid or base), they are always positive. The Ka and Kb values reflect the extent of dissociation of a weak acid or base, but their positive nature directly follows from the equilibrium constant's definition.
-
Solubility Equilibria: Solubility product constants (Ksp) represent the equilibrium between a solid and its dissolved ions. These constants, like all equilibrium constants, are always positive values. A very small Ksp indicates low solubility; a larger Ksp indicates higher solubility but never a negative value.
Practical Implications of the Equilibrium Constant
The equilibrium constant is a powerful tool in chemical calculations and predictions. It allows us to:
-
Predict the direction of a reaction: If the reaction quotient (Q) is less than K, the reaction will proceed to the right (towards products). If Q is greater than K, the reaction will proceed to the left (towards reactants). If Q equals K, the system is at equilibrium.
-
Calculate equilibrium concentrations: Given the initial concentrations and the equilibrium constant, we can calculate the equilibrium concentrations of reactants and products. This often involves solving algebraic equations.
-
Understand the effects of changes in conditions: Le Chatelier's principle states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress. This includes changes in temperature, pressure, concentration, or volume. The equilibrium constant itself changes only with temperature.
-
Design and optimize chemical processes: In industrial processes, the equilibrium constant is crucial for designing reaction conditions that maximize the yield of desired products.
Conclusion: The Non-Negativity of the Equilibrium Constant
In summary, the equilibrium constant K is a critical parameter for understanding and predicting the behavior of reversible chemical reactions. It is always a non-negative value. Any apparent negative value arises from errors in calculation or misinterpretation of related thermodynamic concepts like Gibbs Free Energy. Remembering the fundamental definition of K as a ratio of concentrations (or activities) raised to positive powers eliminates the possibility of a negative value. Concentrations, partial pressures, and activities are always non-negative quantities. Therefore, a negative equilibrium constant is mathematically and physically impossible. A clear understanding of this foundational concept is essential for mastering chemical equilibrium.
Latest Posts
Latest Posts
-
What Is The Optimal Temperature For Amylase
Mar 17, 2025
-
What Is Independent Of The Frequency Of Light
Mar 17, 2025
-
What Does The Arrow In A Chemical Equation Mean
Mar 17, 2025
-
What Is The Measure Of Average Kinetic Energy
Mar 17, 2025
-
Fill In The Blank Unit Circle
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about If The Equilibrium Constant Is Negative What Does That Mean . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
