What Does The Arrow In A Chemical Equation Mean
Muz Play
Mar 17, 2025 · 6 min read
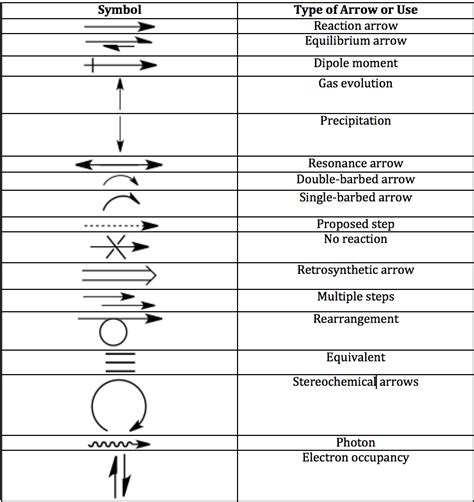
Table of Contents
What Does the Arrow in a Chemical Equation Mean? A Comprehensive Guide
The arrow in a chemical equation is more than just a simple symbol; it's a powerful indicator of the fundamental process of chemical change. Understanding its nuances is crucial for grasping the essence of chemical reactions and predicting their outcomes. This comprehensive guide will delve deep into the meaning of the arrow, exploring its various forms and interpretations within the context of chemical equations.
The Arrow as a Representation of Change
At its most basic level, the arrow (→) in a chemical equation signifies the direction of a chemical reaction. It separates the reactants (the starting materials) from the products (the substances formed as a result of the reaction). The reactants are written on the left side of the arrow, while the products are written on the right. This simple representation encapsulates the fundamental principle of chemistry: matter is neither created nor destroyed, but transformed. The arrow shows this transformation explicitly.
For instance, in the equation:
2H₂ + O₂ → 2H₂O
The arrow indicates that two molecules of hydrogen (H₂) react with one molecule of oxygen (O₂) to produce two molecules of water (H₂O). The arrow visually separates the initial state (reactants) from the final state (products) after the chemical transformation.
Types of Arrows and Their Meanings
While the basic arrow conveys a simple transformation, chemical equations often use variations of the arrow to denote specific aspects of the reaction. Let's explore some of these:
1. The Reversible Reaction Arrow (⇌)
Many chemical reactions are reversible, meaning they can proceed in both the forward and reverse directions. In such cases, a double arrow (⇌) is used to represent this equilibrium. The double arrow indicates that the reactants are forming products simultaneously while the products are reacting to form reactants. The rate of the forward and reverse reactions determines the position of the equilibrium.
Example:
N₂ + 3H₂ ⇌ 2NH₃
This equation represents the Haber-Bosch process for ammonia synthesis. The double arrow signifies that ammonia (NH₃) can decompose back into nitrogen (N₂) and hydrogen (H₂). The relative amounts of reactants and products at equilibrium depend on factors such as temperature and pressure.
2. The Heat of Reaction (ΔH) and its Implications
The arrow can be accompanied by symbols or annotations to provide additional information about the reaction. One crucial piece of information is the heat of reaction (ΔH), which indicates whether the reaction is exothermic (releases heat) or endothermic (absorbs heat). This information is often placed above or below the arrow.
- Exothermic Reaction: If heat is released, a negative ΔH value is indicated, often shown as "-ΔH" above or below the arrow. This signifies that the products have less energy than the reactants.
Example:
CH₄ + 2O₂ → CO₂ + 2H₂O + ΔH
This combustion of methane is exothermic, and the heat released is represented by "+ΔH" .
- Endothermic Reaction: If heat is absorbed, a positive ΔH value is denoted as "+ΔH" above or below the arrow. This indicates that the products have more energy than the reactants.
Example:
N₂ + O₂ + ΔH → 2NO
The formation of nitrogen monoxide (NO) from nitrogen and oxygen is endothermic, requiring energy input.
3. Catalyst Indication
A catalyst speeds up a reaction without being consumed itself. The symbol for a catalyst is often written above the arrow to indicate its presence.
Example:
2H₂O₂ → 2H₂O + O₂ MnO₂
In this decomposition of hydrogen peroxide, manganese dioxide (MnO₂) acts as a catalyst, speeding up the reaction without being permanently altered.
4. Reaction Conditions
The arrow might also have additional information about the reaction conditions, such as temperature (Δ) or pressure (P), written above or below it.
Example:
C(s) + O₂(g) → CO₂(g) Δ
This combustion of carbon indicates that the reaction requires a high temperature (Δ) to proceed efficiently.
5. Multiple Reaction Arrows: Stepwise Reactions
Complex reactions often proceed through multiple steps. In such cases, multiple arrows are used to represent the sequence of intermediate steps leading to the final products.
Example:
A simple multistep reaction might look like this: A → B → C
Here, A reacts to form B, which then reacts to form the final product C. Each arrow represents a separate reaction step.
Beyond the Simple Arrow: Understanding the Implications
The arrow in a chemical equation is not merely a visual separator; it represents the dynamic nature of chemical transformations. It conveys information about the direction of the reaction, the possibility of reversibility, the energy changes involved, the influence of catalysts, and even the specific reaction conditions required. Understanding these nuances is essential for correctly interpreting and predicting the outcome of chemical reactions.
Advanced Applications and Interpretations
The use of arrows in chemical equations extends beyond the basic representation of chemical reactions. In advanced chemistry, the arrow's function becomes even more nuanced:
1. Mechanism Arrows in Organic Chemistry
Organic chemistry often uses curved arrows to illustrate the movement of electrons during a reaction mechanism. These arrows show the flow of electron pairs and help visualize the bond breaking and bond formation steps involved in a reaction. These are distinct from the arrow indicating the overall reaction.
2. Equilibrium Arrows and Equilibrium Constants
The double arrow (⇌) is not just a qualitative indicator of reversibility. It's intrinsically linked to the equilibrium constant (K), a quantitative measure of the relative amounts of reactants and products at equilibrium. A large K indicates that the equilibrium favors the products, while a small K indicates that the equilibrium favors the reactants.
3. Reaction Coordinate Diagrams
In physical chemistry, reaction coordinate diagrams use an arrow to indicate the progress of a reaction along the reaction coordinate, showing energy changes as reactants convert into products. This visual representation clarifies the energy barriers (activation energy) and the overall energy change (ΔH) of the reaction.
4. Rate Laws and Reaction Kinetics
The arrow implicitly connects the rate of the reaction to the concentrations of the reactants. The rate law, which quantitatively describes the relationship between the rate of reaction and reactant concentrations, is determined experimentally and helps in understanding the reaction mechanism.
Conclusion
The arrow in a chemical equation is a deceptively simple symbol that holds a wealth of information about the chemical reaction it represents. It is far more than just a separator; it’s a dynamic indicator conveying the direction of change, energy involved, reaction conditions, reversibility, and even the steps involved in complex reactions. A deep understanding of the arrow's various forms and interpretations is crucial for mastering the principles of chemistry and for effectively communicating chemical processes. Through careful observation of these nuances, we can gain a profound comprehension of the dynamic nature of chemical change and the elegance of chemical transformations. By paying close attention to the arrow and its accompanying notations, we unlock deeper insights into the heart of chemical reactions. This understanding forms the bedrock of advanced chemical concepts and applications, from understanding reaction mechanisms to predicting equilibrium and reaction kinetics.
Latest Posts
Latest Posts
-
What Are The Functions Of The Family
Mar 18, 2025
-
Trends In The Periodic Table Melting Point
Mar 18, 2025
-
What Two Regions Make Up All Atoms
Mar 18, 2025
-
Kinetic Energy Of Simple Harmonic Motion
Mar 18, 2025
-
Delta E And Delta H Relationship
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Does The Arrow In A Chemical Equation Mean . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
