Trends In The Periodic Table Melting Point
Muz Play
Mar 18, 2025 · 6 min read
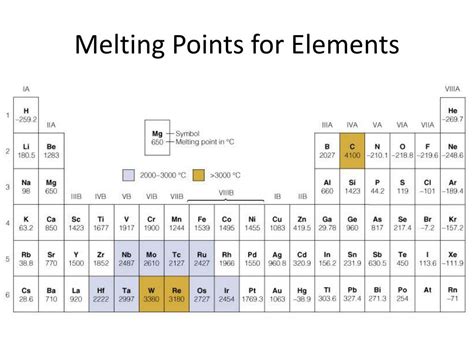
Table of Contents
Trends in Periodic Table Melting Points: A Deep Dive
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. One fascinating property revealing underlying atomic behavior is the melting point – the temperature at which a substance transitions from a solid to a liquid. Understanding trends in melting points across the periodic table provides crucial insights into interatomic forces, bonding characteristics, and crystal structures. This comprehensive exploration delves into these trends, examining the influence of various factors and highlighting exceptions that enrich our understanding of the periodic table's intricate nature.
The Dominant Factors Influencing Melting Points
Several key factors synergistically determine an element's melting point. These include:
1. Atomic Size and Atomic Radius:
Generally, as you move down a group in the periodic table, atomic size increases. Larger atoms have greater distances between their nuclei and valence electrons, resulting in weaker interatomic forces. Consequently, less energy is required to overcome these forces, leading to lower melting points. This trend is clearly visible in Group 1 (alkali metals) and Group 2 (alkaline earth metals). For example, lithium (Li) has a significantly higher melting point than cesium (Cs).
2. Nuclear Charge:
Increased nuclear charge strengthens the electrostatic attraction between the nucleus and valence electrons. This enhanced attraction leads to stronger metallic bonding (in metals) or stronger covalent bonds (in non-metals), resulting in higher melting points. The increase in nuclear charge across a period generally leads to an increase in melting point until the transition metals are reached.
3. Number of Valence Electrons:
The number of valence electrons significantly impacts the strength of metallic bonding. Elements with more valence electrons generally exhibit stronger metallic bonds due to increased electron delocalization. This increased bonding strength translates to higher melting points. This trend is prominently observed across periods involving transition metals. The higher number of valence electrons allows for more extensive electron sharing and stronger metallic bonds.
4. Type of Bonding:
The nature of chemical bonding—metallic, covalent, ionic, or van der Waals—dramatically influences melting points.
-
Metallic Bonding: Metals exhibit strong metallic bonding characterized by delocalized electrons forming a "sea" of electrons surrounding positively charged metal ions. This strong bonding leads to high melting points, particularly in transition metals with multiple valence electrons.
-
Covalent Bonding: Covalent bonds involve the sharing of electron pairs between atoms. The strength of covalent bonds varies significantly depending on the atoms involved, leading to a wide range of melting points. Network covalent solids like diamond and silicon carbide possess extremely high melting points due to the strong and extensive covalent network. Molecular solids with weaker covalent bonds exhibit much lower melting points.
-
Ionic Bonding: Ionic compounds are formed through electrostatic attractions between oppositely charged ions. The strength of ionic bonds depends on the charge of the ions and the distance between them. Generally, compounds with highly charged ions and smaller ionic radii exhibit higher melting points.
-
Van der Waals Forces: These are weak intermolecular forces present in many molecular compounds. They result from temporary fluctuations in electron distribution, creating temporary dipoles. Van der Waals forces are relatively weak, leading to low melting points in substances held together primarily by these forces.
5. Crystal Structure:
The arrangement of atoms or ions in a crystal lattice significantly affects melting points. A well-ordered, tightly packed crystal structure leads to stronger interatomic forces and higher melting points. Conversely, less ordered structures with weaker interatomic interactions exhibit lower melting points. The crystal structure can vary even for the same element under different conditions (allotropy). For instance, carbon exists as diamond (very high melting point) and graphite (relatively low melting point) due to differences in their crystal structures.
Trends Across Periods and Groups
Analyzing trends across periods and groups reveals a nuanced picture influenced by the interplay of the factors discussed above.
Periods (Across a Row):
Generally, melting points tend to increase across a period, especially for the earlier elements. This is primarily attributed to the increase in nuclear charge and the gradual filling of valence orbitals, leading to stronger interatomic interactions. However, this trend is not strictly linear. The transition metals exhibit a more complex trend due to the intricate interplay of factors like d-orbital filling and variations in crystal structure. The trend can also break down towards the end of the period, with non-metals displaying decreasing melting points due to weaker intermolecular forces.
Groups (Down a Column):
Down a group, the general trend is a decrease in melting point. This is largely because atomic size increases, leading to weaker interatomic forces and a greater distance between positively charged nuclei and their surrounding valence electrons. This decrease is particularly pronounced in groups of metals. However, exceptions exist due to the variations in crystal structure and other factors.
Exceptions and Anomalies: Why Some Elements Don't Follow the Trend
While the general trends are helpful, many exceptions challenge the simplistic view. Several factors contribute to these anomalies:
-
Allotropy: The existence of multiple forms (allotropes) of the same element with different crystal structures leads to variations in melting points. A prime example is carbon, with diamond (extremely high melting point) and graphite (relatively low melting point) exhibiting vastly different melting points due to structural differences.
-
Electron Configuration: The specific electron configuration influences bonding strength and thus melting point. Half-filled or fully filled subshells often contribute to higher stability and higher melting points. This is seen in some transition metals.
-
Intermolecular Forces: The strength of intermolecular forces, such as hydrogen bonding, can significantly influence melting points, particularly in non-metallic elements and compounds.
-
Pressure: Pressure significantly affects melting points. High pressure can dramatically increase melting points by bringing atoms closer together, strengthening interatomic forces.
-
Impurities: The presence of impurities can alter the melting point of a substance, usually lowering it.
Applications and Significance
Understanding trends in melting points has numerous practical applications across various fields:
-
Material Science: The selection of materials for specific applications heavily relies on their melting points. High melting point materials are crucial in high-temperature applications, while materials with lower melting points are useful in low-temperature applications.
-
Metallurgy: Melting point is a critical parameter in metallurgy, influencing processes like alloying and casting. Understanding the melting points of metals and their alloys is essential for designing and manufacturing various metal components.
-
Geochemistry: The melting points of minerals play a crucial role in understanding geological processes like rock formation and volcanic eruptions.
-
Chemistry: Melting point determination is a common technique for identifying and characterizing substances in chemical analysis.
Conclusion: A Continuing Exploration
The study of melting point trends in the periodic table offers a rich understanding of the fundamental forces governing the behavior of matter. While general trends exist, exceptions and anomalies highlight the complex interplay of factors influencing this crucial property. Continued research and exploration in this area promise deeper insights into the intricacies of atomic interactions and their influence on material properties, ultimately fueling advancements across various scientific and technological domains. Further research into the effects of pressure, impurities, and the development of more sophisticated computational models will continue to refine our understanding of the nuanced relationship between atomic structure and melting points across the periodic table. The periodic table, with its inherent complexities, remains a source of fascinating discoveries and a testament to the elegant organization of matter in the universe.
Latest Posts
Latest Posts
-
Plant Cell And Animal Cell Similarities
Mar 18, 2025
-
What Is The Total Magnification Of 4x
Mar 18, 2025
-
The Cutaneous Membrane Is Blank To The Muscles
Mar 18, 2025
-
If A Hybrid Offspring Does Not Survive
Mar 18, 2025
-
5 Postulates Of Daltons Atomic Theory
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Trends In The Periodic Table Melting Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
