What Is The Measure Of Average Kinetic Energy
Muz Play
Mar 17, 2025 · 6 min read
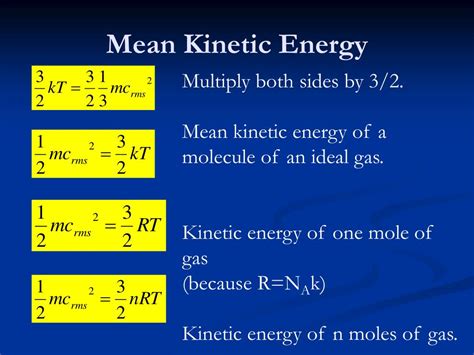
Table of Contents
What is the Measure of Average Kinetic Energy?
Understanding the measure of average kinetic energy is crucial in various fields, from thermodynamics and statistical mechanics to chemistry and material science. It's a fundamental concept that links the microscopic world of atoms and molecules to the macroscopic properties we observe. This article delves deep into this concept, exploring its definition, calculation methods, applications, and its relationship to temperature.
Defining Kinetic Energy
Before we dive into average kinetic energy, let's establish a clear understanding of kinetic energy itself. Kinetic energy is the energy an object possesses due to its motion. It's directly proportional to the object's mass and the square of its velocity. The formula for kinetic energy (KE) is:
KE = 1/2 * mv²
where:
- m represents the mass of the object (in kilograms)
- v represents the velocity of the object (in meters per second)
This formula applies to macroscopic objects, like a rolling ball or a moving car. However, when dealing with microscopic particles like atoms and molecules in gases, liquids, and solids, we need to consider a more nuanced approach. These particles are in constant, random motion, with a wide range of velocities. Therefore, we cannot simply use the formula above for each individual particle and sum them up; instead, we need to consider the average kinetic energy.
Average Kinetic Energy: The Microscopic Perspective
The average kinetic energy of a collection of particles, such as atoms or molecules in a gas, is a measure of their average translational kinetic energy. Translational kinetic energy refers to the energy associated with the movement of the center of mass of a particle. In contrast to rotational or vibrational kinetic energies (which are also relevant for molecules), we are focusing on the kinetic energy purely due to the movement of the particles through space.
The average kinetic energy is directly proportional to the absolute temperature of the system. This relationship is a cornerstone of the kinetic theory of gases and is expressed mathematically as:
KE<sub>avg</sub> = (3/2)kT
where:
- KE<sub>avg</sub> represents the average kinetic energy per particle
- k represents the Boltzmann constant (approximately 1.38 x 10<sup>-23</sup> J/K)
- T represents the absolute temperature of the system (in Kelvin)
This equation signifies a crucial link between the microscopic world (average kinetic energy of particles) and the macroscopic world (temperature). It tells us that as temperature increases, the average kinetic energy of the particles increases proportionally. This explains why hotter substances tend to have faster-moving particles.
Calculating Average Kinetic Energy
Calculating the average kinetic energy depends on the system you are studying:
1. Ideal Gases
For an ideal gas, the average kinetic energy calculation is straightforward, using the equation: KE<sub>avg</sub> = (3/2)kT. This is based on the kinetic theory of gases which assumes that gas particles are point masses, only interacting during collisions, and that these collisions are elastic. The simplicity of this equation makes it valuable in many applications.
2. Non-Ideal Gases and Condensed Phases
In non-ideal gases, liquids, and solids, intermolecular forces become significant. These forces complicate the calculation, making the simple (3/2)kT equation insufficient. The average kinetic energy needs to be determined through more complex methods, often involving statistical mechanics and sophisticated computational techniques like molecular dynamics simulations. These simulations use computational power to track the movement of individual particles and calculate their average kinetic energy based on their interactions.
3. Distribution of Kinetic Energies
It's important to emphasize that while we talk about average kinetic energy, individual particles within a system possess a wide range of kinetic energies. This distribution is described by the Maxwell-Boltzmann distribution, which is a probability distribution showing the likelihood of a particle having a particular kinetic energy at a given temperature. The average kinetic energy represents the mean of this distribution.
Applications of Average Kinetic Energy
The concept of average kinetic energy finds wide applications across various scientific disciplines:
1. Thermodynamics
Average kinetic energy is fundamental to understanding thermodynamic properties like temperature, pressure, and internal energy. The internal energy of a system is directly related to the total kinetic and potential energy of its constituent particles. Changes in temperature are directly linked to changes in the average kinetic energy of the particles.
2. Chemical Kinetics
Reaction rates in chemical reactions are heavily influenced by the average kinetic energy of the reactant molecules. Higher average kinetic energy implies more frequent and energetic collisions, increasing the probability of successful reactions. The activation energy, the minimum energy required for a reaction to occur, is closely related to the average kinetic energy of the molecules.
3. Statistical Mechanics
Statistical mechanics provides a framework for relating microscopic properties of systems (like the average kinetic energy) to macroscopic observable quantities (like pressure and temperature). The average kinetic energy is a key variable in statistical mechanics calculations, allowing predictions of system behavior based on the distribution of energies among the particles.
4. Material Science
The average kinetic energy of atoms within a material influences its physical properties. For instance, the melting point of a solid is related to the average kinetic energy needed to overcome the interatomic forces holding the atoms in a fixed lattice structure. Similarly, diffusion rates in solids are directly related to the average kinetic energy of the diffusing atoms.
5. Astrophysics
In astrophysics, understanding the average kinetic energy of particles in stellar atmospheres and plasmas is crucial for modeling stellar evolution, understanding processes like nuclear fusion, and interpreting observations from telescopes.
Relationship Between Average Kinetic Energy and Temperature
The strong relationship between average kinetic energy and temperature is one of the most important consequences of the kinetic theory of gases. It's a direct proportionality, as shown in the equation KE<sub>avg</sub> = (3/2)kT. This relationship holds for ideal gases but is an approximation for real systems where intermolecular forces influence the energy distribution. This explains why:
- Higher temperatures lead to higher average kinetic energy: As the temperature of a substance increases, its particles move faster, leading to a higher average kinetic energy.
- Lower temperatures lead to lower average kinetic energy: As the temperature decreases, particles slow down, reducing the average kinetic energy. At absolute zero (0 Kelvin), the average kinetic energy theoretically reaches zero, although quantum effects prevent complete cessation of motion.
Conclusion
The measure of average kinetic energy is a fundamental concept that bridges the gap between the microscopic and macroscopic worlds. Its calculation, applications, and relationships with other physical quantities are vital for understanding various physical and chemical phenomena. While the simple (3/2)kT equation provides a good approximation for ideal gases, more complex methods are required for real systems where intermolecular forces are significant. Its importance spans numerous fields, showcasing its enduring relevance in science and engineering. Continued research and advancements in computational methods are crucial for refining our understanding and calculations of average kinetic energy in complex systems.
Latest Posts
Latest Posts
-
Is Boiling A Physical Or Chemical Property
Mar 18, 2025
-
Is Cam Or Kmno4 Better For Alcohols
Mar 18, 2025
-
Metals Metaloids And Nonmetals Which One Has High Lister
Mar 18, 2025
-
How To Find Sampling Distribution Of Sample Mean
Mar 18, 2025
-
Differentiate Between Population Density And Population Distribution
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Measure Of Average Kinetic Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
