Does Gas Have A Definite Shape
Muz Play
Mar 17, 2025 · 6 min read
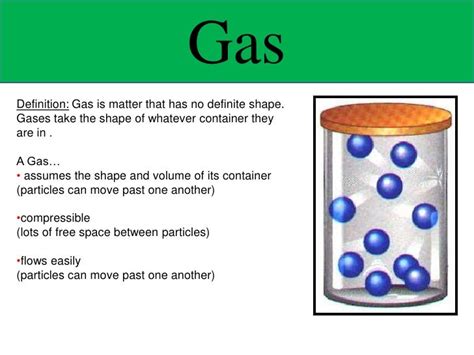
Table of Contents
Does Gas Have a Definite Shape? Exploring the Properties of Gases
Gases, one of the four fundamental states of matter, are known for their unique properties, most notably their lack of a definite shape or volume. Unlike solids and liquids, which maintain a relatively fixed structure, gases readily conform to the shape and volume of their container. This seemingly simple observation opens the door to a fascinating exploration of the microscopic world and the principles of kinetic molecular theory. This article delves deep into the question, "Does gas have a definite shape?", examining the behavior of gas molecules, the factors influencing their movement, and the implications of this characteristic in various scientific fields.
Understanding the Kinetic Molecular Theory of Gases
To understand why gases don't have a definite shape, we need to look at the kinetic molecular theory (KMT). This theory provides a microscopic model to explain the macroscopic properties of gases. The key postulates of the KMT are:
-
Gases are composed of tiny particles: These particles are usually atoms or molecules, far apart relative to their size. This significant intermolecular distance is a crucial factor in the lack of definite shape and volume.
-
These particles are in constant, random motion: They move in straight lines until they collide with each other or the walls of the container. This constant motion is the essence of a gas's lack of fixed structure. The speed of these particles is directly related to the temperature of the gas. Higher temperatures mean faster-moving particles.
-
Collisions between particles and the container walls are elastic: This means that kinetic energy is conserved during collisions. No energy is lost during these interactions, keeping the particles in perpetual motion.
-
The forces of attraction and repulsion between gas particles are negligible: This is especially true for ideal gases, where these intermolecular forces are considered insignificant compared to the kinetic energy of the particles. Real gases show some degree of intermolecular forces, but these forces are generally much weaker than in liquids or solids.
-
The average kinetic energy of gas particles is proportional to the absolute temperature: This means that as the temperature increases, the average kinetic energy of the gas particles also increases, leading to faster movement and more frequent collisions.
Why Gases Don't Have a Definite Shape: The Role of Intermolecular Forces and Kinetic Energy
The absence of a definite shape in gases is a direct consequence of the interplay between the kinetic energy of the gas particles and the weak intermolecular forces. The high kinetic energy of gas particles allows them to overcome the weak attractive forces between them. This freedom of movement enables the gas to expand and fill any available space.
Imagine a gas contained in a balloon. The gas particles are not bound to fixed positions like those in a solid. Instead, they are constantly colliding with each other and the balloon's inner surface. These collisions exert pressure on the balloon, causing it to expand and assume the shape of its container. If you were to transfer the gas to a different shaped container, the gas would readily conform to the new shape. This demonstrates the adaptability and lack of inherent structure in gases.
In contrast: Solids have strong intermolecular forces that hold their particles in a fixed arrangement, giving them a definite shape. Liquids have weaker intermolecular forces, allowing particles to move more freely but still maintain a definite volume. Gases, with their negligible intermolecular forces and high kinetic energy, possess neither a definite shape nor a definite volume.
The Ideal Gas Law and its Implications
The ideal gas law, PV = nRT, encapsulates the relationship between the pressure (P), volume (V), number of moles (n), and temperature (T) of an ideal gas. The constant R is the ideal gas constant. This law perfectly illustrates how a gas adapts to its environment. If the volume of the container changes, the gas will expand or contract to fill the new volume, maintaining a constant pressure (at constant temperature). This adaptation underscores the lack of a defined shape for gases. The gas molecules simply adjust their distribution to occupy the available space.
Deviations from Ideal Gas Behavior: Real Gases
While the ideal gas law provides a useful approximation, real gases deviate from ideal behavior at high pressures and low temperatures. At high pressures, the gas particles are closer together, and the intermolecular forces become more significant, affecting the overall pressure and volume. At low temperatures, the kinetic energy of the particles decreases, making the weak intermolecular forces more influential. These forces can cause the gas to deviate from the ideal gas law's predictions. However, even with these deviations, the fundamental principle remains: real gases still lack a definite shape and will readily adapt to their container's form.
Examples of Gases and their Shape Adaptability
Let's consider a few examples to illustrate this fundamental property of gases:
-
Air: The air we breathe is a mixture of gases (primarily nitrogen and oxygen). It fills our lungs, adopts the shape of a balloon, and spreads throughout a room – all demonstrating the absence of a definite shape.
-
Helium in a balloon: Helium gas, lighter than air, expands to fill the balloon, taking on the balloon's shape. If you were to change the shape of the balloon, the helium would adjust accordingly.
-
Natural gas in a pipeline: Natural gas is transported through extensive pipeline networks. The gas conforms to the shape of the pipes, regardless of their twists and turns.
-
Carbon dioxide in a soda bottle: The carbon dioxide dissolved in a soda bottle is under pressure. When the bottle is opened, the carbon dioxide gas rapidly expands, escaping into the atmosphere, again showcasing its adaptability and lack of defined shape.
Applications and Implications of Gas Shape Adaptability
The lack of a definite shape in gases has significant implications in various fields:
-
Weather patterns: The movement and distribution of gases in the atmosphere, including the mixing of pollutants, are directly related to their ability to adopt the shape of their environment.
-
Industrial processes: Many industrial processes involve the use of gases, such as combustion, chemical reactions, and transportation of materials. Understanding how gases adapt to their containers is essential for optimizing these processes.
-
Medical applications: Medical imaging techniques, such as MRI and CT scans, rely on the behavior of gases in various contexts. Furthermore, the delivery of anesthetic gases to patients also hinges upon their shape-adapting properties.
-
Aerospace engineering: The behavior of gases is critical in the design and operation of aircraft and rockets. Understanding the dynamics of gas flow and pressure is crucial for ensuring safe and efficient flight.
Conclusion: A Definite Answer
The answer to the question, "Does gas have a definite shape?" is a definitive no. Gases, due to the high kinetic energy of their constituent particles and negligible intermolecular forces, lack any inherent structure. They readily adapt to the shape and volume of their container, a characteristic explained by the kinetic molecular theory and further elucidated by the ideal gas law. This fundamental property of gases has profound implications across diverse scientific and engineering disciplines. Understanding this key characteristic is crucial for comprehending various natural phenomena and technological applications. The absence of a definite shape is not a limitation, but rather a defining characteristic of gases that allows them to play such versatile roles in our world.
Latest Posts
Latest Posts
-
Identify The Equation For The Graph
Mar 17, 2025
-
Ions With Positive Charge Are Called
Mar 17, 2025
-
What Is A Derived Unit In Chemistry
Mar 17, 2025
-
What Does The Coefficient Represent In A Chemical Formula
Mar 17, 2025
-
How To Calculate Saturated Vapour Pressure
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Does Gas Have A Definite Shape . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
