What Does The Coefficient Represent In A Chemical Formula
Muz Play
Mar 17, 2025 · 6 min read
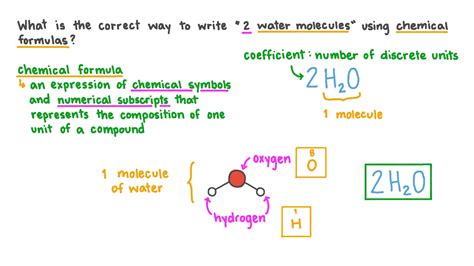
Table of Contents
What Does the Coefficient Represent in a Chemical Formula?
Understanding chemical formulas is fundamental to grasping the principles of chemistry. These formulas, seemingly simple strings of symbols and numbers, convey crucial information about the composition and quantity of substances involved in chemical reactions. While the subscripts within a formula indicate the ratio of atoms within a molecule, the coefficients preceding the formula tell a different, equally important story: they represent the relative number of molecules or moles of a substance participating in a reaction. This article will delve into the meaning and significance of coefficients in chemical formulas, exploring their role in balancing equations and performing stoichiometric calculations.
Understanding Coefficients vs. Subscripts
Before delving into the specifics of coefficients, it's crucial to differentiate them from subscripts. Both are numerical values within a chemical formula, but they represent different aspects:
-
Subscripts: These small numbers written to the lower right of an element's symbol indicate the number of atoms of that element within a single molecule of the compound. For example, in the formula H₂O (water), the subscript '2' signifies that there are two hydrogen atoms for every one oxygen atom in a single water molecule.
-
Coefficients: These numbers are written to the left of a chemical formula. They indicate the number of molecules (or moles, a unit used to quantify amounts of substances in chemistry) of that entire compound involved in a reaction. They represent the relative proportions of reactants and products in a balanced chemical equation.
Let's illustrate the difference with an example:
2H₂O
In this expression, '2' is the coefficient. It indicates two molecules of water. Each water molecule still contains two hydrogen atoms and one oxygen atom (as indicated by the subscripts). Therefore, this expression represents a total of 4 hydrogen atoms and 2 oxygen atoms.
The Importance of Coefficients in Balanced Chemical Equations
The Law of Conservation of Mass dictates that matter cannot be created or destroyed in a chemical reaction; only rearranged. This principle is reflected in balanced chemical equations. Coefficients are essential for achieving this balance: they ensure that the number of atoms of each element is the same on both the reactant (left-hand side) and product (right-hand side) sides of the equation.
Consider the reaction between hydrogen and oxygen to form water:
H₂ + O₂ → H₂O (Unbalanced)
This equation is unbalanced because there are two oxygen atoms on the reactant side and only one on the product side. To balance it, we use coefficients:
2H₂ + O₂ → 2H₂O (Balanced)
Now, there are four hydrogen atoms and two oxygen atoms on both sides of the equation, fulfilling the Law of Conservation of Mass. The coefficient '2' before H₂ indicates two molecules of hydrogen gas, and the coefficient '2' before H₂O indicates two molecules of water.
Coefficients and Stoichiometric Calculations
Coefficients in balanced chemical equations are the cornerstone of stoichiometry, the branch of chemistry dealing with quantitative relationships between reactants and products in chemical reactions. They allow us to perform calculations relating the amounts of substances involved.
For instance, in the balanced equation above:
2H₂ + O₂ → 2H₂O
The coefficients tell us that two moles of hydrogen react with one mole of oxygen to produce two moles of water. This ratio is crucial for determining how much of a product can be formed from a given amount of reactant (theoretical yield) or how much reactant is needed to produce a desired amount of product.
Example Stoichiometry Calculation:
Let's say we want to calculate the amount of water produced when 4 moles of hydrogen react with sufficient oxygen. From the balanced equation, we know the mole ratio of H₂ to H₂O is 2:2, which simplifies to 1:1. Therefore, if 4 moles of hydrogen react, 4 moles of water will be produced.
Coefficients and Limiting Reactants
In many real-world scenarios, reactants aren't present in the exact stoichiometric ratios indicated by the balanced equation. One reactant will be completely consumed before the others, limiting the amount of product formed. This reactant is called the limiting reactant.
Coefficients help us identify the limiting reactant. By comparing the mole ratios of reactants to their actual quantities, we can determine which reactant will be used up first. The limiting reactant dictates the maximum amount of product that can be formed.
Example with Limiting Reactant:
Let's consider the reaction:
N₂ + 3H₂ → 2NH₃
Suppose we have 2 moles of N₂ and 6 moles of H₂. According to the balanced equation, 1 mole of N₂ requires 3 moles of H₂. In this case, we have enough H₂ (6 moles) to react completely with the available N₂ (2 moles). Therefore, N₂ is the limiting reactant, and the amount of NH₃ produced will be determined by the amount of N₂ available.
Coefficients and Molar Mass Calculations
Coefficients, combined with molar mass (the mass of one mole of a substance), enable us to perform mass-to-mass stoichiometry calculations. These calculations allow us to convert the mass of a reactant or product to the mass of another substance involved in the reaction.
For example, if we know the mass of a reactant and its molar mass, we can calculate the number of moles. Using the coefficients from the balanced equation, we can then determine the number of moles of another substance and finally convert this to its mass using its molar mass.
Coefficients and Gas Stoichiometry
Coefficients are particularly useful when dealing with gaseous reactants and products. According to Avogadro's Law, equal volumes of gases at the same temperature and pressure contain the same number of molecules. This means that the coefficients in a balanced chemical equation also represent the relative volumes of gases involved in a reaction, provided they are at the same temperature and pressure.
This simplifies gas stoichiometry calculations because we can directly use the volume ratios from the balanced equation, avoiding the need for molar mass conversions in some cases.
Advanced Applications of Coefficients: Equilibrium and Rate Laws
Beyond basic stoichiometry, coefficients play a subtle yet important role in more advanced areas of chemistry:
-
Chemical Equilibrium: In reversible reactions, the coefficients appear in the equilibrium constant expression, influencing the position of equilibrium. The equilibrium constant relates the concentrations of reactants and products at equilibrium, and the coefficients determine the exponents in this expression.
-
Rate Laws: While not directly involved in the rate law itself (which is experimentally determined), the stoichiometric coefficients provide a starting point for understanding the relationship between reactant concentrations and reaction rate. In some cases, the reaction order might correspond to the stoichiometric coefficient, though this is not always the case.
Conclusion
Coefficients in chemical formulas are far more than simple numbers; they are fundamental tools for understanding and quantifying chemical reactions. Their crucial role in balancing equations, performing stoichiometric calculations (including those involving limiting reactants, molar masses, and gases), and contributing to our understanding of chemical equilibrium and reaction rates highlights their profound importance in chemistry. Mastering the meaning and application of coefficients is essential for anyone seeking to fully grasp the principles and practical applications of this vital scientific field. From basic stoichiometry to advanced chemical concepts, the coefficient remains a cornerstone of quantitative chemical analysis.
Latest Posts
Latest Posts
-
What Are The 3 Parts Of An Rna Nucleotide
Mar 18, 2025
-
When Do You Use Parentheses In Writing A Chemical Formula
Mar 18, 2025
-
Which Process Takes Place In Chloroplasts
Mar 18, 2025
-
How To Do A Slope Field
Mar 18, 2025
-
How Many Electrons Can The D Orbital Hold
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Does The Coefficient Represent In A Chemical Formula . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
