What Is A Derived Unit In Chemistry
Muz Play
Mar 17, 2025 · 6 min read
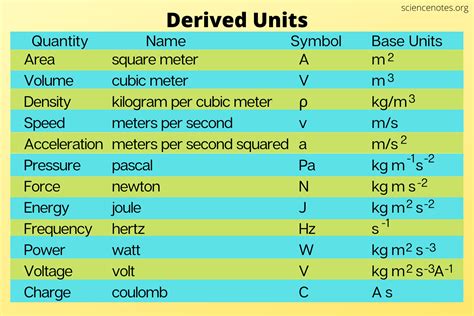
Table of Contents
What is a Derived Unit in Chemistry? A Comprehensive Guide
Derived units are a cornerstone of scientific measurement, providing a standardized way to quantify various properties in chemistry. Understanding them is crucial for accurately representing experimental data, interpreting chemical processes, and communicating findings effectively within the scientific community. This comprehensive guide will delve into the intricacies of derived units in chemistry, exploring their definition, common examples, practical applications, and the importance of their consistent use.
Understanding Fundamental and Derived Units
Before diving into the specifics of derived units, it’s essential to understand the distinction between fundamental and derived units. Fundamental units are the basic building blocks of measurement, defined independently and not expressed in terms of other units. In the International System of Units (SI), the seven fundamental units are:
- Meter (m): The base unit of length.
- Kilogram (kg): The base unit of mass.
- Second (s): The base unit of time.
- Ampere (A): The base unit of electric current.
- Kelvin (K): The base unit of thermodynamic temperature.
- Mole (mol): The base unit of amount of substance.
- Candela (cd): The base unit of luminous intensity.
Derived units, on the other hand, are formed by combining fundamental units using mathematical operations such as multiplication, division, and exponentiation. They represent properties that are not directly measured but are derived from combinations of fundamental quantities. This system of units provides a coherent and interconnected framework for all scientific measurements, ensuring consistency and reproducibility across experiments.
Common Derived Units in Chemistry
Numerous derived units play vital roles in various chemical applications. Here are some of the most frequently encountered examples:
1. Volume (m³)
Volume, a measure of the three-dimensional space occupied by a substance, is a classic example of a derived unit. It's derived from the fundamental unit of length (meter):
- Cubic meter (m³): Represents a cube with sides of one meter each. Smaller units like cubic centimeters (cm³) and liters (L) are commonly used in chemistry. The relationship between these units is crucial for accurate calculations: 1 m³ = 10⁶ cm³ = 1000 L.
Practical Application: Determining the volume of a solution, gas, or solid is essential in various chemical processes, from stoichiometric calculations to reaction kinetics studies.
2. Density (kg/m³)
Density, the ratio of mass to volume, expresses how much mass is packed into a given volume. It's a derived unit combining mass and volume:
- Kilograms per cubic meter (kg/m³): Represents the mass (in kilograms) of a substance per unit volume (in cubic meters). Grams per cubic centimeter (g/cm³) is another commonly used unit.
Practical Application: Density is critical for identifying unknown substances, determining the purity of samples, and understanding the behavior of materials under different conditions.
3. Velocity (m/s)
Velocity, a measure of the rate of change of displacement, is derived from the fundamental units of length and time:
- Meters per second (m/s): Represents the distance (in meters) traveled per unit time (in seconds).
Practical Application: In chemical kinetics, the velocity of a chemical reaction can be determined by measuring the rate of change of reactant or product concentrations over time.
4. Acceleration (m/s²)
Acceleration, the rate of change of velocity, combines length and time:
- Meters per second squared (m/s²): Represents the change in velocity (in meters per second) per unit time (in seconds).
Practical Application: While less frequently used directly in chemistry compared to other derived units, understanding acceleration is crucial for analyses involving movement of particles or fluids in chemical processes.
5. Force (N = kg⋅m/s²)
Force, the product of mass and acceleration, is a derived unit crucial in various chemical contexts:
- Newton (N): Defined as the force required to accelerate a mass of one kilogram at one meter per second squared.
Practical Application: Studying forces acting on molecules or particles, especially in areas like physical chemistry and spectroscopy, necessitates understanding the Newton.
6. Pressure (Pa = N/m² = kg⋅m⁻¹⋅s⁻²)
Pressure, the force exerted per unit area, is a derived unit vital in many chemical processes:
- Pascal (Pa): Represents one newton of force per square meter. Atmospheres (atm) and millimeters of mercury (mmHg) are also frequently used units in chemistry.
Practical Application: Pressure plays a critical role in many chemical reactions, particularly those involving gases, and influences reaction rates and equilibria.
7. Energy (J = kg⋅m²/s²)
Energy, the capacity to do work, is a fundamental concept in chemistry and is expressed using a derived unit:
- Joule (J): One joule is equal to the work done when a force of one newton is applied over a distance of one meter. Kilojoules (kJ) are often used in chemical contexts.
Practical Application: Energy changes are at the heart of chemical reactions, thermodynamics, and spectroscopy. Calculations involving enthalpy, entropy, and activation energy rely heavily on the joule.
8. Power (W = J/s = kg⋅m²/s³)
Power, the rate at which work is done or energy is transferred, is another essential derived unit:
- Watt (W): One watt is equal to one joule per second.
Practical Application: Studying reaction rates, particularly those that are temperature-dependent or involve energy transfer, frequently utilizes the watt.
9. Concentration (mol/L or mol/m³)
Concentration, a crucial aspect of solutions and mixtures in chemistry, is a derived unit combining amount of substance and volume:
- Moles per liter (mol/L) or molarity (M): Represents the amount of substance (in moles) dissolved in one liter of solution. Moles per cubic meter (mol/m³) is the SI unit.
Practical Application: Molarity is ubiquitous in chemistry, determining reactant ratios, controlling reaction rates, and analyzing solution compositions.
10. Molar Mass (kg/mol)
Molar mass, the mass of one mole of a substance, combines mass and amount of substance:
- Kilograms per mole (kg/mol): Represents the mass (in kilograms) of one mole of a substance. Grams per mole (g/mol) is commonly used.
Practical Application: Molar mass is fundamental for stoichiometric calculations, relating the mass of reactants and products in a chemical reaction.
Importance of Consistent Use of Derived Units
The consistent use of derived units, based on the SI system, is paramount for several reasons:
- Reproducibility: Standardized units ensure that experiments can be replicated and results compared accurately across different laboratories and researchers.
- Clarity and Communication: Using consistent units avoids ambiguity and facilitates clear communication of scientific findings within the scientific community.
- Error Reduction: Correct unit usage minimizes errors in calculations and interpretations of data.
- Interoperability: Standardized units enable the integration and exchange of data across different scientific disciplines and technologies.
Conclusion
Derived units represent a vital tool in chemistry, enabling precise quantification of various properties that are essential for understanding and manipulating chemical processes. Their consistent use based on the SI system is crucial for ensuring reproducibility, clarity, and accuracy in chemical research and applications. Mastering the understanding and application of these units is essential for any aspiring or practicing chemist. By thoroughly understanding these foundational concepts, scientists can confidently conduct experiments, analyze data, and contribute to the advancement of chemical knowledge. The correct application of derived units underpins the entire structure of chemical measurement, paving the way for accurate data analysis and consistent interpretations. The relationship between these units, as demonstrated through conversion factors, ensures that experimental results can be easily compared and interpreted regardless of the specific units used in the measurements. The continued emphasis on using these units accurately is crucial for maintaining the rigor and reproducibility of chemical research.
Latest Posts
Latest Posts
-
An Indians Looking Glass For The White Man Year
Mar 17, 2025
-
Protons Neutrons And Electrons For Helium
Mar 17, 2025
-
Magnetic Field In A Bar Magnet
Mar 17, 2025
-
Competes With Substrate For Binding To An Active Site
Mar 17, 2025
-
Which Compound Is Soluble In Water
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is A Derived Unit In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
