Subatomic Particle With A Negative Charge
Muz Play
Mar 17, 2025 · 6 min read
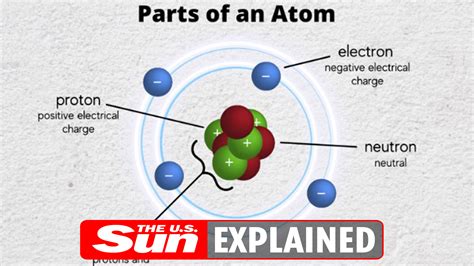
Table of Contents
The Electron: A Deep Dive into the Negatively Charged Subatomic Particle
The universe, in all its magnificent complexity, is built from fundamental building blocks. Among these, the electron stands out as a pivotal player, a subatomic particle carrying a single unit of negative electric charge. Understanding the electron is crucial to comprehending the nature of matter, electricity, chemistry, and the very fabric of reality. This comprehensive article explores the electron's properties, history, significance, and ongoing research surrounding this fascinating particle.
Discovering the Electron: A Journey Through History
While the concept of electricity had been known for centuries, the understanding of its fundamental nature remained elusive until the late 19th century. The discovery of the electron is largely attributed to J.J. Thomson, who, through his experiments with cathode ray tubes in 1897, provided compelling evidence for the existence of a negatively charged particle much smaller than an atom.
Thomson's Cathode Ray Experiments
Thomson's ingenious experiments involved passing an electric current through a vacuum tube. He observed that rays, later named cathode rays, emanated from the negative electrode (cathode) and traveled towards the positive electrode (anode). By applying electric and magnetic fields, he demonstrated that these rays were composed of negatively charged particles. Importantly, he found that the charge-to-mass ratio of these particles was independent of the material of the cathode, suggesting that these particles were fundamental constituents of all matter. This groundbreaking discovery revolutionized our understanding of the atom, proving it was not indivisible as previously thought.
Millikan's Oil Drop Experiment: Quantifying the Charge
While Thomson established the existence of the electron and determined its charge-to-mass ratio, the precise value of its charge remained unknown. This crucial piece of the puzzle was provided by Robert Millikan through his famous oil drop experiment in 1909. By carefully observing the motion of electrically charged oil droplets in an electric field, Millikan was able to measure the charge on each droplet. Crucially, he found that the charge was always a multiple of a fundamental unit, confirming the discrete nature of electric charge and providing a precise measurement of the elementary charge, the charge of a single electron.
Properties of the Electron: A Subatomic Profile
The electron is a fundamental particle, meaning it is not composed of smaller constituents. It is classified as a lepton, a family of elementary particles that participate in weak interactions and electromagnetic interactions but not in strong interactions. Here's a summary of its key properties:
- Charge: -1.602 x 10^-19 Coulombs (this is the elementary charge, often denoted as -e)
- Mass: 9.109 x 10^-31 kilograms (approximately 1/1836 the mass of a proton)
- Spin: 1/2 (a quantum property related to intrinsic angular momentum)
- Lepton Number: +1 (a conserved quantum number in particle interactions)
- Antiparticle: Positron (a particle with the same mass but opposite charge)
Wave-Particle Duality: A Quantum Enigma
One of the most remarkable aspects of the electron is its wave-particle duality. This concept, central to quantum mechanics, postulates that the electron exhibits properties of both a wave and a particle. This duality is not merely a theoretical concept; it's experimentally verifiable through phenomena like electron diffraction, where electrons exhibit wave-like behavior by diffracting through a crystal lattice, creating interference patterns. This dual nature highlights the limitations of classical physics in describing the behavior of subatomic particles.
The Electron's Role in Atoms and Molecules
Electrons play a crucial role in determining the chemical and physical properties of atoms and molecules. They occupy specific energy levels or orbitals surrounding the atom's nucleus, governed by the laws of quantum mechanics. The arrangement of electrons in these orbitals determines an atom's chemical reactivity and its ability to form bonds with other atoms.
Atomic Structure and Electron Shells
The electrons in an atom are arranged in shells, each representing a specific energy level. The innermost shell can hold up to two electrons, while subsequent shells can accommodate progressively more electrons. The number of electrons in the outermost shell, called the valence shell, determines the atom's valency—its ability to form chemical bonds. Atoms tend to react with other atoms to achieve a stable electron configuration, usually a full outermost shell.
Chemical Bonding: The Electron's Glue
Chemical bonds are formed through the interaction of electrons between atoms. There are several types of chemical bonds, including:
- Ionic Bonds: Involve the transfer of electrons from one atom to another, creating ions with opposite charges that attract each other.
- Covalent Bonds: Involve the sharing of electrons between atoms, creating a stable molecular structure.
- Metallic Bonds: Involve the delocalization of electrons across a lattice of metal atoms, contributing to the characteristic properties of metals, such as conductivity.
Electrons in Technology: Powering Our Modern World
The electron's unique properties have made it indispensable to numerous technological advancements. Its ability to carry charge and move freely forms the basis of countless technologies shaping our modern world:
Electronics and Semiconductors: The Heart of Modern Devices
The controlled flow of electrons is the foundation of modern electronics. Semiconductors, materials with conductivity between conductors and insulators, are engineered to control electron flow in transistors and integrated circuits, the building blocks of computers, smartphones, and countless other electronic devices. By manipulating the electron's movement in semiconductors, we can process information, amplify signals, and perform complex computations.
Electricity and Magnetism: Harnessing Electron Flow
Electricity, the flow of electrons through a conductor, powers our homes, industries, and transportation systems. Similarly, magnetism is closely intertwined with electron motion. Electric currents generate magnetic fields, and changing magnetic fields induce electric currents—principles exploited in electric motors, generators, and transformers.
Medical Imaging and Treatment: Seeing Inside and Fighting Disease
Electrons are crucial in medical imaging techniques like electron microscopy and X-ray technology. Electron microscopes allow for visualizing structures at the nanoscale, while X-rays, produced by accelerating electrons, enable us to see inside the human body without surgery. Furthermore, electrons play a role in radiation therapy, utilizing high-energy electron beams to target and destroy cancerous cells.
Ongoing Research and Future Directions
Despite our extensive knowledge about the electron, research continues to unravel its deeper mysteries. Current research focuses on areas such as:
Quantum Computing: Exploiting Electron Spin
Researchers are exploring the possibility of using electron spin for quantum computation. Electron spin can exist in two states, representing 0 and 1 in a qubit, the fundamental unit of quantum information. Harnessing the quantum properties of electrons holds the potential for creating incredibly powerful computers capable of solving complex problems beyond the reach of classical computers.
Investigating the Electron's Anomalous Magnetic Moment
The electron's magnetic moment, a measure of its magnetic strength, is slightly different from the value predicted by the Standard Model of particle physics. This discrepancy, known as the electron's anomalous magnetic moment, suggests the existence of unknown particles or interactions. Ongoing high-precision experiments are attempting to resolve this discrepancy, potentially leading to breakthroughs in our understanding of fundamental physics.
Conclusion: The Electron's Enduring Significance
The electron, a seemingly tiny particle, holds immense significance in our understanding of the universe and its technological applications. From the fundamental structure of matter to the intricacies of modern electronics, the electron’s influence is profound and pervasive. Ongoing research continues to deepen our understanding of this fundamental particle, promising further breakthroughs in science and technology that will shape the future. The journey of understanding the electron is far from over; it remains a vibrant area of research, pushing the boundaries of our knowledge and enriching our technological capabilities. The electron's enduring significance is a testament to the power of fundamental scientific inquiry and its capacity to transform the world.
Latest Posts
Latest Posts
-
What Are The Three Basic Components Of An Atom
Mar 17, 2025
-
Does Gas Have A Definite Shape
Mar 17, 2025
-
If The Equilibrium Constant Is Negative What Does That Mean
Mar 17, 2025
-
How Does An Atom Become A Cation
Mar 17, 2025
-
Which Body Cavity Protects The Spinal Column
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Subatomic Particle With A Negative Charge . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
