How To Use The Activity Series
Muz Play
Mar 15, 2025 · 5 min read
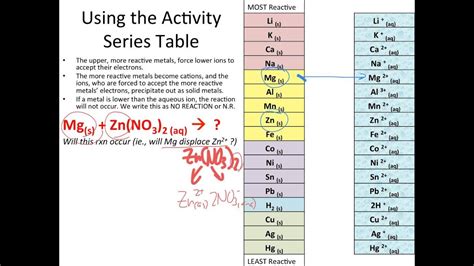
Table of Contents
How to Use the Activity Series: A Comprehensive Guide
The activity series, also known as the reactivity series, is a crucial tool in chemistry for predicting the outcome of single displacement reactions. Understanding how to use it effectively is essential for both students and professionals alike. This comprehensive guide will break down the activity series, explaining its construction, applications, and limitations, with plenty of examples to solidify your understanding.
What is the Activity Series?
The activity series is a list of metals (and sometimes nonmetals) arranged in order of their decreasing reactivity. Reactivity refers to the tendency of an element to lose electrons and undergo oxidation. Elements higher on the series are more reactive than those lower down. This means they more readily lose electrons to form positive ions.
This series isn't simply a random arrangement; it's based on experimental observations of how different elements behave in redox reactions (reactions involving the transfer of electrons). By observing which elements displace others from compounds, chemists have established this hierarchical order.
Understanding the Arrangement: Metals and Their Reactivity
The activity series typically lists metals, starting with the most reactive at the top and proceeding to the least reactive at the bottom. A simplified version might look like this:
Li > K > Ba > Sr > Ca > Na > Mg > Al > Mn > Zn > Fe > Cd > Co > Ni > Sn > Pb > H > Cu > Ag > Hg > Au
Key elements to note:
- Li (Lithium) to Au (Gold): This represents a spectrum of reactivity. Lithium is highly reactive, readily reacting with water and even air. Gold, on the other hand, is extremely unreactive, resisting most chemical attacks.
- H (Hydrogen): The inclusion of hydrogen is important. Metals above hydrogen in the series will react with acids (like HCl or H₂SO₄) to produce hydrogen gas. Metals below hydrogen will not. This is a key application of the series.
- Noble Metals: Elements like gold (Au), silver (Ag), and platinum (Pt) are often termed "noble metals" due to their low reactivity.
How to Use the Activity Series: Predicting Single Displacement Reactions
The primary application of the activity series is in predicting the outcome of single displacement reactions. These reactions follow a general pattern:
A + BC → AC + B
Where:
- A is a more reactive element.
- B is a less reactive element.
- BC is a compound.
- AC is a new compound formed.
The Rule: A single displacement reaction will only occur if element A is more reactive than element B. In other words, A must be higher on the activity series than B.
Let's look at some examples:
Example 1: Reaction of Zinc with Hydrochloric Acid
Zinc (Zn) is higher on the activity series than hydrogen (H). Therefore, zinc will displace hydrogen from hydrochloric acid (HCl):
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
This reaction produces zinc chloride and hydrogen gas.
Example 2: Reaction of Copper with Hydrochloric Acid
Copper (Cu) is below hydrogen on the activity series. Therefore, it will not displace hydrogen from hydrochloric acid:
Cu(s) + HCl(aq) → No Reaction
No reaction will occur because copper is less reactive than hydrogen.
Example 3: Reaction of Iron with Copper(II) Sulfate
Iron (Fe) is higher on the activity series than copper (Cu). Thus, iron will displace copper from copper(II) sulfate:
Fe(s) + CuSO₄(aq) → FeSO₄(aq) + Cu(s)
This reaction produces iron(II) sulfate and solid copper.
Example 4: Reaction of Silver with Iron(III) Chloride
Silver (Ag) is lower on the activity series than iron (Fe). Therefore, it will not displace iron from iron(III) chloride:
Ag(s) + FeCl₃(aq) → No Reaction
No reaction will occur.
Beyond Single Displacement: Other Applications
While primarily used for single displacement reactions, the activity series provides insights into other chemical phenomena:
- Corrosion: The activity series helps predict the susceptibility of metals to corrosion. More reactive metals corrode more readily.
- Electrochemical Cells: The relative positions of metals in the activity series influence the voltage generated in electrochemical cells (batteries).
- Metal Extraction: The activity series guides the choice of methods used to extract metals from their ores. Less reactive metals can be extracted using simpler methods, while more reactive metals require more energy-intensive processes.
Limitations of the Activity Series
While a valuable tool, the activity series has some limitations:
- Simplified Representation: It's a simplified representation of complex chemical behavior. Reaction conditions (temperature, concentration, presence of catalysts) can influence the outcome.
- Not All Elements Included: The activity series primarily focuses on metals, with limited inclusion of nonmetals.
- Qualitative, Not Quantitative: It provides a qualitative assessment of reactivity, not a precise quantitative measure.
Expanding Your Knowledge: Exploring Further
To deepen your understanding of the activity series, consider these points:
- Investigate standard reduction potentials: Standard reduction potentials offer a more quantitative measure of reactivity, complementing the activity series.
- Explore different versions of the activity series: Variations of the activity series exist, some including nonmetals or offering more detailed information.
- Conduct experiments: Performing simple experiments involving single displacement reactions can provide firsthand experience in applying the activity series.
Conclusion: Mastering the Activity Series for Chemical Predictions
The activity series is an essential tool in chemistry, providing a straightforward method for predicting the outcome of single displacement reactions and offering insights into other chemical processes. While it has limitations, understanding its construction and applications empowers you to confidently interpret chemical reactions and make informed predictions. By grasping the fundamentals and exploring the related concepts discussed here, you can master the activity series and elevate your understanding of chemical reactivity. Remember to practice using the activity series with various examples to solidify your understanding and build your problem-solving skills in chemistry. Through consistent practice and further exploration, you will confidently navigate the world of chemical reactions.
Latest Posts
Latest Posts
-
Boiling Point On Graph In Celsius
Mar 15, 2025
-
List The Classification Levels From Broadest To Most Specific
Mar 15, 2025
-
Equipments For Measuring Volume Of Acids
Mar 15, 2025
-
The Acid Test Tells Whether A Mineral Is Called
Mar 15, 2025
-
Definition Contour Integral Union Of Curves
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How To Use The Activity Series . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
