How To Write Compounds In Chemistry
Muz Play
Mar 31, 2025 · 6 min read
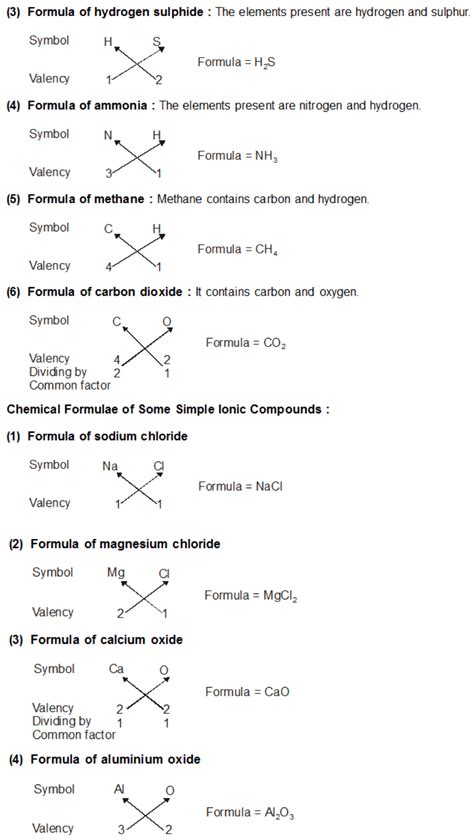
Table of Contents
How to Write Compounds in Chemistry: A Comprehensive Guide
Chemistry, at its core, is the study of matter and its interactions. Understanding how to write chemical compounds is fundamental to communicating effectively within this field. This comprehensive guide will delve into the intricacies of chemical nomenclature, providing you with a robust understanding of how to correctly represent various types of compounds. We'll cover everything from simple binary compounds to complex organic molecules, equipping you with the tools to confidently write and interpret chemical formulas.
Understanding Chemical Formulas
A chemical formula is a concise way of representing the composition of a chemical compound using chemical symbols and numerical subscripts. The subscripts indicate the number of atoms of each element present in one molecule of the compound. For example, H₂O represents one molecule of water, containing two hydrogen atoms and one oxygen atom.
Key Components of Chemical Formulas:
-
Chemical Symbols: These are abbreviations for elements (e.g., H for hydrogen, O for oxygen, C for carbon). You'll need to be familiar with the periodic table to understand these symbols.
-
Subscripts: These numbers written slightly below and to the right of a chemical symbol indicate the number of atoms of that element in the compound. If no subscript is written, it is understood to be 1.
-
Parentheses: Parentheses are used to group atoms together, especially in polyatomic ions. A subscript outside the parentheses applies to all atoms within the parentheses.
Writing Ionic Compounds
Ionic compounds are formed through the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). Naming and writing ionic compounds follow specific rules:
1. Identifying the Cations and Anions:
First, determine the cation and anion present. Metals generally form cations, while nonmetals usually form anions. Transition metals can form multiple cations with different charges (oxidation states), requiring Roman numerals to specify the charge.
2. Writing the Formula:
The formula is written with the cation first, followed by the anion. The charges of the ions must balance to create a neutral compound. This often involves finding the least common multiple of the charges to determine the subscripts.
Example: Sodium Chloride (NaCl)
- Sodium (Na) forms a +1 cation (Na⁺).
- Chlorine (Cl) forms a -1 anion (Cl⁻).
- The charges balance (1+ + 1- = 0), so the formula is NaCl.
Example: Magnesium Oxide (MgO)
- Magnesium (Mg) forms a +2 cation (Mg²⁺).
- Oxygen (O) forms a -2 anion (O²⁻).
- The charges balance (2+ + 2- = 0), so the formula is MgO.
Example: Iron(III) Oxide (Fe₂O₃)
- Iron (Fe) can form multiple cations. Iron(III) indicates a +3 charge (Fe³⁺).
- Oxygen (O) forms a -2 anion (O²⁻).
- To balance the charges, we need two Fe³⁺ ions and three O²⁻ ions: 2(3+) + 3(2-) = 0. Therefore, the formula is Fe₂O₃.
Writing Covalent Compounds
Covalent compounds are formed when atoms share electrons to achieve a stable electron configuration. Naming and writing covalent compounds, also known as molecular compounds, utilize prefixes to indicate the number of atoms of each element.
1. Identifying the Elements:
Determine the elements involved in the covalent bond.
2. Using Prefixes:
Prefixes are used to indicate the number of atoms of each element. Common prefixes include:
- Mono- (1)
- Di- (2)
- Tri- (3)
- Tetra- (4)
- Penta- (5)
- Hexa- (6)
- Hepta- (7)
- Octa- (8)
- Nona- (9)
- Deca- (10)
The prefix "mono-" is often omitted for the first element unless it is necessary to distinguish between different compounds.
3. Writing the Formula:
The less electronegative element is written first, followed by the more electronegative element. The prefixes indicate the number of atoms of each element.
**Example: Carbon Dioxide (CO₂) **
- Carbon (C) is less electronegative than oxygen (O).
- One carbon atom and two oxygen atoms are present.
- The formula is CO₂. (Note: "mono-" is omitted for carbon).
Example: Dinitrogen Tetroxide (N₂O₄)
- Two nitrogen atoms and four oxygen atoms are present.
- The formula is N₂O₄.
Writing Compounds with Polyatomic Ions
Polyatomic ions are groups of atoms that carry a net charge. These ions behave similarly to monatomic ions in the formation of ionic compounds.
1. Recognizing Polyatomic Ions:
You'll need to familiarize yourself with common polyatomic ions such as:
- Nitrate (NO₃⁻)
- Sulfate (SO₄²⁻)
- Phosphate (PO₄³⁻)
- Ammonium (NH₄⁺)
- Hydroxide (OH⁻)
- Carbonate (CO₃²⁻)
2. Balancing Charges:
The charges of the cation and the polyatomic anion must balance to form a neutral compound.
Example: Ammonium Nitrate (NH₄NO₃)
- Ammonium (NH₄⁺) has a +1 charge.
- Nitrate (NO₃⁻) has a -1 charge.
- The charges balance, resulting in the formula NH₄NO₃.
Example: Calcium Phosphate [Ca₃(PO₄)₂]
- Calcium (Ca²⁺) has a +2 charge.
- Phosphate (PO₄³⁻) has a -3 charge.
- To balance the charges, we need three Ca²⁺ ions and two PO₄³⁻ ions: 3(2+) + 2(3-) = 0. The formula is Ca₃(PO₄)₂. Note the use of parentheses to indicate that two phosphate ions are present.
Writing Hydrates
Hydrates are compounds that contain water molecules within their crystal structure. The number of water molecules is indicated using a dot followed by a numerical prefix.
Example: Copper(II) Sulfate Pentahydrate (CuSO₄·5H₂O)
- Copper(II) sulfate (CuSO₄) has five water molecules associated with it.
- The formula is CuSO₄·5H₂O.
Writing Organic Compounds
Organic chemistry deals with carbon-containing compounds. The naming and writing of organic compounds are more complex and follow systematic rules defined by IUPAC (International Union of Pure and Applied Chemistry) nomenclature. A deep dive into organic nomenclature is beyond the scope of this introductory guide, but understanding basic functional groups and their representation is crucial.
Example: Methane (CH₄)
The simplest organic compound, methane, contains one carbon atom bonded to four hydrogen atoms.
Tips for Writing Chemical Compounds Accurately
-
Know the Periodic Table: Familiarity with the periodic table is essential for identifying elements and their symbols.
-
Understand Oxidation States: This is particularly important for transition metals, which can exhibit multiple oxidation states.
-
Memorize Common Polyatomic Ions: Knowing common polyatomic ions will significantly simplify the process of writing ionic compounds.
-
Practice: The best way to master chemical nomenclature is through consistent practice. Work through numerous examples to solidify your understanding.
-
Use Online Resources: Many online resources and educational websites offer interactive exercises and quizzes to help you practice writing chemical formulas.
Conclusion
Writing chemical compounds accurately is a cornerstone of chemical communication. By understanding the rules for ionic compounds, covalent compounds, compounds with polyatomic ions, hydrates, and the basics of organic nomenclature, you'll be well-equipped to confidently represent the composition of various chemical substances. Remember to practice consistently to reinforce your knowledge and build proficiency in this fundamental aspect of chemistry. With dedication and consistent effort, you can master the art of writing chemical compounds and confidently navigate the world of chemical formulas.
Latest Posts
Latest Posts
-
What Is The Chromosomal Basis Of Inheritance
Apr 02, 2025
-
Two Different Ionic Compounds Each Contain
Apr 02, 2025
-
Part Ii Equilibria Involving Sparingly Soluble Salts
Apr 02, 2025
-
Adding Strong Acid To A Buffer
Apr 02, 2025
-
The Most Reactive Group In The Periodic Table
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How To Write Compounds In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
