Hydrogen Bonds Can Be Broken By
Muz Play
Mar 23, 2025 · 6 min read
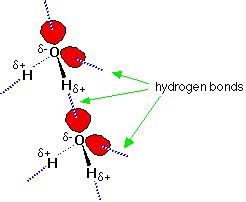
Table of Contents
Hydrogen Bonds Can Be Broken By: A Comprehensive Guide
Hydrogen bonds, a crucial type of intermolecular force, play a pivotal role in shaping the structure and function of biological molecules and materials. Understanding how these bonds are formed and, equally importantly, how they are broken is essential across various scientific disciplines. This comprehensive guide delves into the various factors and methods that can disrupt hydrogen bonds, exploring their implications in chemistry, biology, and beyond.
The Nature of Hydrogen Bonds
Before examining how hydrogen bonds are broken, let's briefly revisit their nature. A hydrogen bond is a special type of dipole-dipole attraction between molecules, not a covalent bond within a molecule. It occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a nearby molecule. This creates a relatively strong electrostatic interaction, significantly influencing the properties of substances like water.
The strength of a hydrogen bond is significantly weaker than a covalent bond but stronger than other intermolecular forces like van der Waals forces. This relatively moderate strength is what makes hydrogen bonds so dynamic and susceptible to disruption.
Factors that Break Hydrogen Bonds
Several factors can influence the breaking of hydrogen bonds. These can be broadly classified into those that affect the energy of the bond and those that physically disrupt the molecular arrangement:
1. Temperature
Increased temperature is arguably the most common way to break hydrogen bonds. Higher temperatures provide molecules with more kinetic energy. This increased energy allows molecules to overcome the attractive forces holding them together in hydrogen bonds. The molecules move more vigorously, disrupting the relatively weak electrostatic interactions. This is readily observable in the transition of ice (a highly hydrogen-bonded structure) to liquid water and then to steam.
2. pH Changes
Changes in pH significantly affect the ability of molecules to form hydrogen bonds. The pH of a solution dictates the concentration of hydrogen ions (H⁺) and hydroxide ions (OH⁻). These ions can interact with molecules participating in hydrogen bonds, disrupting the existing interactions. For instance, in acidic conditions, the excess H⁺ ions can compete for the electronegative atoms involved in hydrogen bonds, weakening or breaking them. Similarly, basic conditions with excess OH⁻ ions can also interfere with hydrogen bonding.
3. Solvents
The presence of polar solvents can disrupt hydrogen bonds. Polar solvents, like water itself, can form their own hydrogen bonds with the molecules involved in the original hydrogen bonds. This competition for hydrogen bonding partners effectively weakens and breaks the existing interactions. The ability of a solvent to disrupt hydrogen bonds is often related to its polarity and its ability to form its own hydrogen bonds. For example, water is a very effective hydrogen bond breaker, whereas non-polar solvents like hexane have minimal impact.
Non-polar solvents, conversely, are unable to form hydrogen bonds and will have little impact on existing hydrogen bonds. They can, however, influence the arrangement of molecules indirectly by altering the hydrophobic interactions which may be supporting a hydrogen-bonded structure.
4. Pressure
Increased pressure can also disrupt hydrogen bonds, although this effect is less pronounced than temperature changes. High pressure forces molecules closer together, which can disrupt the optimal geometry required for effective hydrogen bonding. The compression can lead to steric hindrance, preventing the formation of stable hydrogen bonds.
5. Mechanical Forces (Shear Stress)
Mechanical forces, such as shear stress, can physically disrupt hydrogen bonds. These forces directly alter the spatial arrangement of molecules, breaking the hydrogen bonds that rely on a specific molecular orientation. This is particularly relevant in materials science, where the mechanical properties of materials are strongly influenced by hydrogen bonding.
6. Chemical Modification
Chemical modification of the molecules involved in hydrogen bonding can effectively break or prevent their formation. Modifying a molecule such that the electronegative atom is no longer available or its electronegativity is altered can significantly disrupt hydrogen bonding. This approach is commonly employed in biochemistry and drug design to alter the properties and interactions of molecules. For example, introducing a bulky group near a hydrogen bond donor might sterically hinder the formation of the bond.
7. Chaotropic Agents
Chaotropic agents are substances that disrupt the order of water molecules and, consequently, hydrogen bonds. These agents, such as guanidinium chloride or urea, can intercalate between water molecules, disrupting the intricate network of hydrogen bonds that water molecules form with each other. This disruption can indirectly lead to the breaking of hydrogen bonds in other molecules within the solution.
Implications of Breaking Hydrogen Bonds
The ability to break hydrogen bonds has far-reaching implications across various fields:
Biology
-
Protein folding and unfolding: Hydrogen bonds play a crucial role in stabilizing the secondary, tertiary, and quaternary structures of proteins. Breaking these bonds through changes in temperature or pH can lead to protein denaturation, altering its function. This process is vital in many biological processes, including enzyme activity and cellular regulation.
-
DNA replication and transcription: Hydrogen bonds between complementary base pairs in DNA are essential for its double helix structure and stability. The breaking of these bonds is crucial during DNA replication and transcription, enabling the separation of strands and the access of enzymes to the genetic information.
-
Enzyme-substrate interactions: The specific binding of enzymes to their substrates often relies on hydrogen bonds. Breaking these bonds can interrupt the catalytic process, affecting the overall reaction rate.
-
Cell membrane stability: The fluidity and permeability of cell membranes are influenced by hydrogen bonds between lipid molecules. Disrupting these bonds can alter the membrane's properties and its ability to regulate the passage of substances.
Chemistry
-
Solubility of compounds: The solubility of many compounds in water is dependent on their ability to form hydrogen bonds with water molecules. Breaking hydrogen bonds can impact the solubility of these compounds.
-
Phase transitions: Phase transitions, such as melting and boiling, are directly related to the breaking of intermolecular forces, including hydrogen bonds. The strength of hydrogen bonds determines the temperature at which these transitions occur.
-
Polymer properties: The properties of many polymers are influenced by hydrogen bonding between polymer chains. Breaking these bonds can affect the flexibility, strength, and other mechanical properties of the polymer.
Materials Science
-
Material strength and stability: Hydrogen bonds play a crucial role in the strength and stability of various materials, including biomaterials and certain polymers. Understanding how to break or reinforce these bonds is vital in designing materials with specific properties.
-
Self-assembly of materials: The self-assembly of materials often involves hydrogen bonds, which drive the organization of molecules into specific structures. Controlling the breaking of these bonds allows for the manipulation of self-assembly processes.
Methods to Break Hydrogen Bonds: A Summary
The methods and factors discussed earlier can be summarized as follows:
- Thermal methods: Increasing the temperature to provide sufficient kinetic energy to overcome the hydrogen bond's energy barrier.
- Chemical methods: Modifying the molecules involved, introducing chaotropic agents, or changing the pH to disrupt the bond's electrostatic interactions.
- Physical methods: Applying pressure or mechanical forces to disrupt the molecular arrangement and break the hydrogen bonds.
- Solvent methods: Using appropriate polar solvents that compete for hydrogen bonding interactions, thereby weakening the existing bonds.
Understanding how hydrogen bonds are broken is fundamental to comprehending a vast array of phenomena in chemistry, biology, and materials science. The ability to control and manipulate hydrogen bonds opens up avenues for developing new materials, designing advanced drugs, and gaining a deeper understanding of biological processes. Further research into the intricacies of hydrogen bond disruption promises exciting advancements in these and other fields.
Latest Posts
Latest Posts
-
Absolute Value And Order Of Operations
Mar 25, 2025
-
How To Calculate The Heat Capacity Of The Calorimeter
Mar 25, 2025
-
What Is The Relationship Between Temperature And Pressure
Mar 25, 2025
-
Is Toxicity A Physical Or Chemical Property
Mar 25, 2025
-
Plasma Membrane In A Plant Cell
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Hydrogen Bonds Can Be Broken By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
