Identify The Compound With Ionic Bonds
Muz Play
Mar 15, 2025 · 6 min read
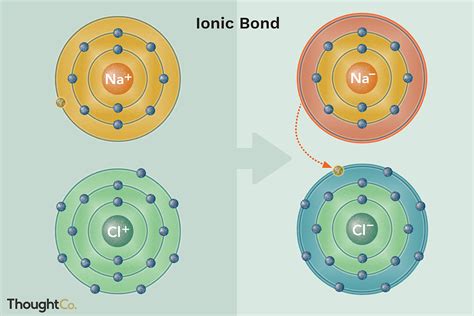
Table of Contents
Identifying Compounds with Ionic Bonds: A Comprehensive Guide
Ionic bonds, the electrostatic forces of attraction between oppositely charged ions, form the backbone of a vast array of compounds. Understanding how to identify these compounds is crucial in chemistry, impacting our understanding of material properties, chemical reactions, and biological processes. This comprehensive guide will delve into the intricacies of ionic bonding, providing you with the knowledge and tools to confidently identify compounds held together by these powerful bonds.
Understanding Ionic Bonds: The Fundamentals
Before we embark on identifying ionic compounds, let's solidify our understanding of the fundamental principles governing ionic bonding. Ionic bonds arise from the electrostatic attraction between a cation (a positively charged ion) and an anion (a negatively charged ion). This charge difference stems from the transfer of electrons from one atom to another, typically between a metal and a non-metal.
The Electron Transfer Process
The process involves a metal atom, which readily loses electrons to achieve a stable electron configuration (often resembling a noble gas), and a non-metal atom, which readily gains electrons to achieve the same stable configuration. This transfer of electrons creates ions with opposite charges, leading to the strong electrostatic attraction that constitutes the ionic bond.
Example: Consider the formation of sodium chloride (NaCl), common table salt. Sodium (Na), a metal, readily loses one electron to become a positively charged sodium ion (Na⁺). Chlorine (Cl), a non-metal, readily gains one electron to become a negatively charged chloride ion (Cl⁻). The electrostatic attraction between Na⁺ and Cl⁻ forms the ionic bond in NaCl.
Factors Influencing Ionic Bond Formation
Several factors influence the formation and strength of ionic bonds:
-
Electronegativity Difference: A significant difference in electronegativity between the atoms is crucial. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. A large difference indicates that one atom will more readily attract electrons, leading to electron transfer and ion formation.
-
Ionization Energy: The energy required to remove an electron from an atom (ionization energy) influences the ease with which a metal loses electrons. Metals with low ionization energies readily form cations.
-
Electron Affinity: The energy change associated with gaining an electron (electron affinity) indicates how readily a non-metal gains electrons to form an anion. Non-metals with high electron affinities readily form anions.
-
Lattice Energy: The strength of the ionic bond is also reflected in the lattice energy, the energy released when gaseous ions combine to form a solid crystal lattice. Higher lattice energy indicates a stronger ionic bond.
Identifying Ionic Compounds: Key Characteristics and Methods
Identifying ionic compounds requires a systematic approach, utilizing various characteristics and methods. While no single characteristic definitively proves an ionic bond, a combination of observations and analyses provides strong evidence.
1. Examining the Constituent Elements
The most straightforward approach involves looking at the constituent elements. Ionic compounds are typically formed between a metal and a non-metal. This is a strong indicator, but not a foolproof rule, as some exceptions exist (e.g., some compounds with polyatomic ions).
Examples:
- NaCl (Sodium Chloride): Sodium (Na) is an alkali metal, and chlorine (Cl) is a halogen. Their combination strongly suggests an ionic compound.
- MgO (Magnesium Oxide): Magnesium (Mg) is an alkaline earth metal, and oxygen (O) is a non-metal. This combination also points towards an ionic compound.
- Al₂O₃ (Aluminum Oxide): Aluminum (Al) is a metal, and oxygen (O) is a non-metal. Again, this points towards an ionic bond.
2. Analyzing Physical Properties
Ionic compounds exhibit characteristic physical properties:
-
High Melting and Boiling Points: The strong electrostatic forces in ionic bonds require significant energy to overcome, resulting in high melting and boiling points. This is in contrast to covalent compounds, which typically have much lower melting and boiling points.
-
Crystalline Structure: Ionic compounds usually form well-ordered crystalline structures, reflecting the regular arrangement of ions in the crystal lattice. This contributes to their often brittle nature.
-
Solubility in Polar Solvents: Ionic compounds are often soluble in polar solvents like water, due to the interaction between the charged ions and the polar solvent molecules.
-
Electrical Conductivity: Ionic compounds are typically good conductors of electricity when molten (liquid) or dissolved in solution, as the ions are free to move and carry charge. In their solid state, however, they are generally poor conductors. This is because the ions are held rigidly in the crystal lattice and are not free to move.
3. Considering the Chemical Formula
The chemical formula can provide valuable clues. The formula typically shows the ratio of cations to anions in the compound, reflecting the charge balance achieved through electron transfer. The presence of a metal cation and a non-metal anion further strengthens the indication of an ionic compound.
Examples:
-
CaCl₂ (Calcium Chloride): The formula indicates one calcium ion (Ca²⁺) for every two chloride ions (Cl⁻), maintaining charge neutrality.
-
K₂O (Potassium Oxide): Two potassium ions (K⁺) balance the charge of one oxide ion (O²⁻).
-
Al₂(SO₄)₃ (Aluminum Sulfate): While containing a polyatomic ion (sulfate), the presence of a metal cation (Al³⁺) and a polyatomic anion still indicates an ionic character.
4. Employing Spectroscopic Techniques
Advanced techniques like X-ray diffraction can analyze the crystal structure, providing definitive evidence of the regular arrangement of ions characteristic of ionic compounds. Other spectroscopic techniques can provide information about the bonding, further confirming the ionic nature. These methods are commonly used in research settings.
Exceptions and Complex Cases
While the guidelines above are generally reliable, some exceptions and complexities warrant consideration:
Polar Covalent Compounds
Some compounds exhibit characteristics intermediate between purely ionic and purely covalent bonding. These polar covalent compounds have significant differences in electronegativity between atoms, but not enough for complete electron transfer. They possess partial ionic character, reflected in dipole moments.
Polyatomic Ions
Compounds containing polyatomic ions (ions composed of multiple atoms) can exhibit ionic bonding. The interaction between the metal cation and the polyatomic anion is still primarily ionic, even though covalent bonds exist within the polyatomic ion itself. Examples include ammonium chloride (NH₄Cl) and potassium nitrate (KNO₃).
Metallic Bonding
While not strictly ionic, metallic bonding involves the delocalization of electrons across a lattice of metal atoms. It differs significantly from ionic bonding in that there is no distinct transfer of electrons to form ions.
Conclusion: A Multifaceted Approach to Identification
Identifying compounds with ionic bonds is not a simple yes-or-no process. It requires a multifaceted approach that considers the constituent elements, physical properties, chemical formula, and in some cases, advanced spectroscopic analysis. By understanding the underlying principles of ionic bonding and applying these identification methods, you can confidently analyze the nature of chemical compounds and their bonding interactions. Remember that while a single characteristic might suggest an ionic bond, a combination of observations provides the strongest evidence. This comprehensive guide provides a robust foundation for accurately identifying the ionic nature of compounds across a range of chemical systems.
Latest Posts
Latest Posts
-
What Is The Difference Between Hunger And Appetite
Mar 15, 2025
-
Boiling Point On Graph In Celsius
Mar 15, 2025
-
List The Classification Levels From Broadest To Most Specific
Mar 15, 2025
-
Equipments For Measuring Volume Of Acids
Mar 15, 2025
-
The Acid Test Tells Whether A Mineral Is Called
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Identify The Compound With Ionic Bonds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
