Identify The Functional Group In Each Compound
Muz Play
Mar 28, 2025 · 8 min read
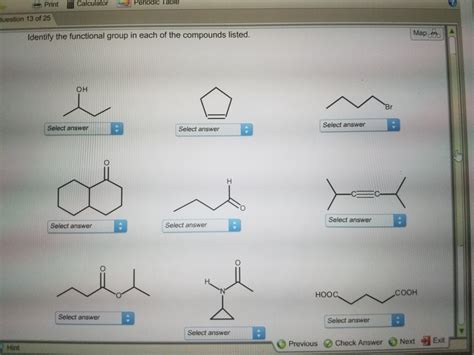
Table of Contents
Identifying Functional Groups in Organic Compounds: A Comprehensive Guide
Organic chemistry, the study of carbon-containing compounds, is built upon the concept of functional groups. These are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of that molecule. Identifying these functional groups is crucial for understanding the properties and reactivity of organic compounds. This comprehensive guide will delve into various functional groups, providing detailed explanations and examples to help you master this fundamental aspect of organic chemistry.
Understanding Functional Groups
A functional group is a specific atom or group of atoms within a molecule that exhibits a characteristic set of chemical reactions. These groups dictate the molecule's behavior, influencing its physical properties like boiling point, melting point, and solubility, as well as its chemical reactivity. Recognizing functional groups allows chemists to predict how a molecule will react and to design synthetic pathways to create new molecules.
The presence of a particular functional group defines the class of organic compounds. For example, all alcohols contain the hydroxyl (-OH) group, while all ketones contain the carbonyl group (C=O) bonded to two carbon atoms. This classification system allows for systematic organization and prediction of reactivity within organic chemistry.
Major Functional Groups and Their Identification
Let's explore some of the most common functional groups, their structures, and how to identify them within a molecule.
1. Hydrocarbons: The Foundation
Hydrocarbons are the simplest organic compounds, containing only carbon and hydrogen atoms. They serve as the backbone for many other functional groups. There are three main types:
-
Alkanes: These contain only single carbon-carbon bonds. They are saturated hydrocarbons, meaning they have the maximum number of hydrogen atoms attached to each carbon atom. Examples include methane (CH₄), ethane (C₂H₆), and propane (C₃H₈). Identifying alkanes involves looking for chains of carbon atoms with only single bonds and hydrogen atoms filling the remaining valencies.
-
Alkenes: These contain at least one carbon-carbon double bond (C=C). They are unsaturated hydrocarbons. Examples include ethene (C₂H₄) and propene (C₃H₆). Identifying alkenes involves recognizing the presence of a double bond between two carbon atoms.
-
Alkynes: These contain at least one carbon-carbon triple bond (C≡C). They are also unsaturated hydrocarbons. Examples include ethyne (C₂H₂) and propyne (C₃H₄). Identifying alkynes is similar to identifying alkenes, but instead of a double bond, you look for a triple bond between two carbon atoms.
2. Oxygen-Containing Functional Groups
Oxygen is a highly electronegative atom, and its presence significantly influences the reactivity of organic molecules. Several important functional groups contain oxygen:
-
Alcohols (-OH): The hydroxyl group (-OH) is characteristic of alcohols. The -OH group is bonded to a carbon atom. Examples include methanol (CH₃OH) and ethanol (CH₃CH₂OH). Identifying alcohols involves looking for an -OH group directly attached to a carbon atom (not an oxygen or another heteroatom).
-
Ethers (R-O-R'): Ethers contain an oxygen atom bonded to two carbon atoms (R and R' can be the same or different alkyl groups). Examples include diethyl ether (CH₃CH₂OCH₂CH₃) and methyl propyl ether (CH₃OCH₂CH₂CH₃). Identifying ethers involves recognizing an oxygen atom bonded to two carbon atoms.
-
Aldehydes (R-CHO): Aldehydes have a carbonyl group (C=O) at the end of a carbon chain. The carbonyl carbon is bonded to one carbon atom and one hydrogen atom. Examples include formaldehyde (HCHO) and acetaldehyde (CH₃CHO). Identifying aldehydes involves searching for a carbonyl group at the terminal position of a carbon chain.
-
Ketones (R-CO-R'): Ketones also contain a carbonyl group (C=O), but unlike aldehydes, the carbonyl carbon is bonded to two carbon atoms. Examples include acetone (CH₃COCH₃) and butanone (CH₃CH₂COCH₃). Identifying ketones involves finding a carbonyl group within a carbon chain, not at the end.
-
Carboxylic Acids (-COOH): Carboxylic acids possess a carboxyl group (-COOH), which is a combination of a carbonyl group and a hydroxyl group. These are acidic compounds. Examples include acetic acid (CH₃COOH) and formic acid (HCOOH). Identifying carboxylic acids involves looking for the -COOH group.
-
Esters (R-COO-R'): Esters are formed by the reaction of a carboxylic acid and an alcohol. They contain a carbonyl group bonded to an oxygen atom, which is further bonded to a carbon atom. Examples include ethyl acetate (CH₃COOCH₂CH₃) and methyl benzoate (C₆H₅COOCH₃). Identifying esters involves recognizing the -COO- linkage between two carbon atoms.
3. Nitrogen-Containing Functional Groups
Nitrogen is another important element in organic chemistry, forming several key functional groups:
-
Amines (R-NH₂, R₂NH, R₃N): Amines contain a nitrogen atom bonded to one, two, or three carbon atoms (or hydrogen atoms). They are classified as primary (R-NH₂), secondary (R₂NH), or tertiary (R₃N) amines depending on the number of carbon atoms bonded to the nitrogen. Examples include methylamine (CH₃NH₂), dimethylamine ((CH₃)₂NH), and trimethylamine ((CH₃)₃N). Identifying amines involves recognizing a nitrogen atom with bonds to carbon and/or hydrogen atoms.
-
Amides (R-CONH₂): Amides contain a carbonyl group (C=O) bonded to a nitrogen atom. They are derivatives of carboxylic acids. Examples include acetamide (CH₃CONH₂) and benzamide (C₆H₅CONH₂). Identifying amides involves looking for the -CONH₂ group.
-
Nitriles (R-CN): Nitriles contain a cyano group (-CN), which is a carbon atom triple-bonded to a nitrogen atom. Examples include acetonitrile (CH₃CN) and benzonitrile (C₆H₅CN). Identifying nitriles involves recognizing the -CN group.
4. Sulfur-Containing Functional Groups
Sulfur, similar to oxygen, can form several important functional groups:
-
Thiols (-SH): Thiols, also known as mercaptans, contain a sulfhydryl group (-SH) which is analogous to the hydroxyl group (-OH) in alcohols. They have a characteristic foul odor. Examples include methanethiol (CH₃SH) and ethanethiol (CH₃CH₂SH). Identifying thiols involves searching for an -SH group bonded to a carbon atom.
-
Sulfides (R-S-R'): Sulfides are sulfur analogs of ethers, with a sulfur atom bonded to two carbon atoms. Examples include dimethyl sulfide (CH₃SCH₃) and diethyl sulfide (CH₃CH₂SCH₂CH₃). Identifying sulfides involves finding a sulfur atom bonded to two carbon atoms.
5. Halogen-Containing Functional Groups
Halogens (fluorine, chlorine, bromine, and iodine) can be present as substituents in organic molecules:
- Haloalkanes (R-X): Haloalkanes contain a halogen atom (X = F, Cl, Br, I) bonded to a carbon atom. Examples include chloromethane (CH₃Cl) and bromomethane (CH₃Br). Identifying haloalkanes involves recognizing the presence of a halogen atom directly bonded to a carbon atom.
Strategies for Identifying Functional Groups
Identifying functional groups often requires a systematic approach:
-
Identify the carbon skeleton: Start by identifying the main carbon chain or ring system.
-
Look for heteroatoms: Identify atoms other than carbon and hydrogen (e.g., oxygen, nitrogen, sulfur, halogens). These atoms are often part of functional groups.
-
Recognize characteristic bonding patterns: Look for double bonds (C=C, C=O), triple bonds (C≡C, C≡N), and characteristic groupings like -OH, -COOH, -NH₂, -CN, etc.
-
Consider the context: The location of a functional group within the molecule can significantly affect its reactivity. For example, a carbonyl group in an aldehyde will react differently than a carbonyl group in a ketone.
-
Use spectroscopic techniques: Advanced techniques like Infrared (IR) spectroscopy, Nuclear Magnetic Resonance (NMR) spectroscopy, and Mass Spectrometry (MS) can confirm the presence and structure of functional groups.
Examples of Identifying Functional Groups in Complex Molecules
Let's analyze a few examples to solidify your understanding:
Example 1: CH₃CH₂CH₂COOH
This molecule contains a carboxylic acid functional group (-COOH).
Example 2: CH₃CH=CHCH₂OH
This molecule contains both an alkene (C=C) and an alcohol (-OH) functional group.
Example 3: CH₃COOCH₂CH₃
This molecule contains an ester (-COO-) functional group.
Example 4: CH₃CH₂NH₂
This molecule contains a primary amine (-NH₂) functional group.
Example 5: C₆H₅CHO
This molecule contains an aldehyde (-CHO) functional group (the benzene ring is a substituent).
Conclusion
Identifying functional groups is a cornerstone of organic chemistry. Understanding the structure and reactivity of these groups is essential for predicting the properties and behavior of organic compounds. By mastering this skill, you'll be well-equipped to understand complex chemical reactions and design synthetic pathways to create new and useful molecules. Remember to approach the identification systematically, looking for characteristic atoms, bonding patterns, and groups, and consider the context within the larger molecule. This comprehensive guide provides a solid foundation for your journey into the fascinating world of organic chemistry. Practice identifying functional groups in various molecules to strengthen your understanding and build your expertise. Remember to consult textbooks and other learning resources for further exploration and practice problems. The more you practice, the more proficient you will become at identifying functional groups in a wide variety of organic compounds.
Latest Posts
Latest Posts
-
Moment Of Inertia Of Uniform Rod
Mar 31, 2025
-
Keratin And Collagen Are Examples Of Which Class Of Proteins
Mar 31, 2025
-
Job Order Costing Vs Process Costing
Mar 31, 2025
-
What Is The Chemical Equation For Aerobic Respiration
Mar 31, 2025
-
Equation Of The Tangent Line Implicit Differentiation
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Identify The Functional Group In Each Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
