Identifying Acids And Bases By Their Chemical Formula
Muz Play
Mar 27, 2025 · 6 min read
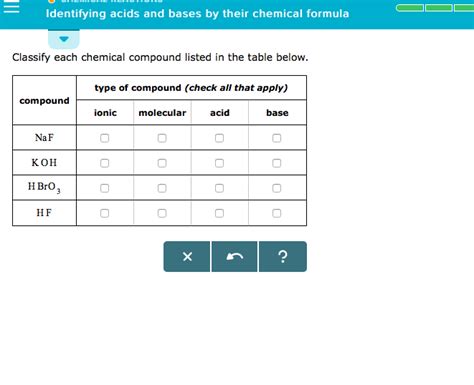
Table of Contents
Identifying Acids and Bases by Their Chemical Formula
Identifying acids and bases solely from their chemical formulas is a fundamental skill in chemistry. While pH measurements and indicators provide experimental ways to determine acidity or basicity, understanding the underlying chemical structure from the formula offers a quicker and more insightful approach. This article will delve into various methods and rules to accurately identify acids and bases based on their chemical formulas, covering both Arrhenius, Brønsted-Lowry, and Lewis definitions.
Arrhenius Definition: A Simple Starting Point
The Arrhenius definition, while the simplest, provides a good foundation for identifying acids and bases. According to Arrhenius:
- Acid: An acid is a substance that produces hydrogen ions (H⁺) when dissolved in water.
- Base: A base is a substance that produces hydroxide ions (OH⁻) when dissolved in water.
Therefore, looking at a chemical formula, if you see an H at the beginning of the formula, it's a strong indicator of an acid. Similarly, the presence of OH often denotes a base. However, this is a simplification, and many exceptions exist.
Examples of Arrhenius Acids and Bases
- HCl (Hydrochloric Acid): Clearly shows an H at the beginning, making it an acid. In water, it dissociates into H⁺ and Cl⁻ ions.
- HNO₃ (Nitric Acid): Another example, the presence of H indicates acidic nature. It dissociates into H⁺ and NO₃⁻ ions in water.
- NaOH (Sodium Hydroxide): The presence of OH clearly indicates it's a base. It dissociates into Na⁺ and OH⁻ ions in water.
- KOH (Potassium Hydroxide): Similar to NaOH, the OH group signifies its basicity. It dissociates into K⁺ and OH⁻ ions in water.
Brønsted-Lowry Definition: A Broader Perspective
The Brønsted-Lowry definition expands the scope of acids and bases. It defines them based on proton (H⁺) transfer:
- Acid: A Brønsted-Lowry acid is a proton donor.
- Base: A Brønsted-Lowry base is a proton acceptor.
This definition doesn't restrict acids and bases to aqueous solutions, making it more versatile than the Arrhenius definition. Identifying acids and bases using this definition requires examining the potential of a molecule to either donate or accept a proton.
Identifying Brønsted-Lowry Acids and Bases from Formulas
The ability to donate or accept a proton is often related to the presence of certain functional groups. For example:
- Carboxylic Acids (RCOOH): The -COOH group readily donates a proton, making carboxylic acids Brønsted-Lowry acids. Examples include acetic acid (CH₃COOH) and formic acid (HCOOH).
- Amines (RNH₂): Amines contain the -NH₂ group, which can accept a proton, thus acting as Brønsted-Lowry bases. Examples include methylamine (CH₃NH₂) and ammonia (NH₃).
- Ammonium Salts (RNH₃⁺): Ammonium salts, possessing the -NH₃⁺ group, can act as Brønsted-Lowry acids by donating a proton.
Amphoteric Substances: Acting as Both Acid and Base
Some substances can act as both proton donors and acceptors, depending on the reaction conditions. These are called amphoteric substances. Water (H₂O) is a classic example. It can act as an acid by donating a proton to a stronger base, or as a base by accepting a proton from a stronger acid. Other examples include bicarbonate ion (HCO₃⁻) and hydrogen sulfate ion (HSO₄⁻). Their formulas do not immediately reveal their amphoteric nature; their behavior depends on the reaction environment.
Lewis Definition: The Electron Pair Perspective
The Lewis definition provides the broadest perspective on acids and bases, focusing on electron pairs rather than proton transfer:
- Lewis Acid: A Lewis acid is an electron pair acceptor.
- Lewis Base: A Lewis base is an electron pair donor.
This definition significantly expands the range of substances considered acids and bases. Many substances that don't fit the Arrhenius or Brønsted-Lowry definitions readily qualify as Lewis acids or bases.
Identifying Lewis Acids and Bases from Formulas
Identifying Lewis acids and bases from formulas often requires understanding the electronic structure and the presence of electron-deficient or electron-rich centers.
- Electron-deficient species: Molecules or ions with incomplete octets often act as Lewis acids because they can accept electron pairs to achieve a stable octet. Examples include boron trifluoride (BF₃) and aluminum chloride (AlCl₃). Their formulas suggest electron deficiency due to the low number of surrounding atoms.
- Species with lone pairs of electrons: Molecules or ions possessing lone pairs of electrons readily donate those pairs, acting as Lewis bases. Examples include ammonia (NH₃), water (H₂O), and hydroxide ion (OH⁻). Their formulas indicate the presence of lone pairs on the central atom.
- Transition Metal Ions: Transition metal ions, due to their empty d orbitals, often act as Lewis acids, readily accepting electron pairs from Lewis bases to form coordinate covalent bonds.
The Lewis definition is the most encompassing, including all substances that fit the Arrhenius and Brønsted-Lowry definitions and many more.
Beyond Simple Formulas: Considering Structure and Context
While the presence of H, OH, or specific functional groups provides initial clues, accurately classifying a substance as an acid or base often requires considering its complete structure and the context of the reaction.
- Polyprotic Acids: Acids capable of donating multiple protons, like sulfuric acid (H₂SO₄) and phosphoric acid (H₃PO₄), require careful consideration of each proton's ionization strength. Their formulas clearly suggest multiple acidic protons, but the relative strengths of each proton must be assessed individually.
- Organic Molecules: Complex organic molecules may possess multiple functional groups, leading to more intricate acid-base behavior. For example, amino acids contain both a carboxylic acid group (-COOH) and an amino group (-NH₂), leading to zwitterionic behavior.
- Reaction Context: The behavior of a substance can vary depending on the reaction environment. A weak acid in one reaction might act as a strong acid in another reaction with a different base.
Therefore, simply looking at a chemical formula isn't always sufficient. A deeper understanding of the molecular structure and the specific reaction conditions is essential for accurate classification.
Practical Applications and Importance
The ability to identify acids and bases from their chemical formulas is crucial in various chemical applications:
- Predicting Reaction Outcomes: Knowing the acidic or basic nature of reactants helps predict the products and the direction of a reaction.
- Designing Chemical Processes: This knowledge is paramount in designing chemical processes, such as synthesis, separations, and purifications.
- Understanding Biological Systems: Many biological processes rely on acid-base reactions, and identifying acidic and basic components in biological molecules is critical for understanding these processes.
- Environmental Monitoring: Monitoring acidity and basicity is vital in environmental studies and pollution control.
Conclusion: A Multifaceted Approach
Identifying acids and bases using chemical formulas is a multifaceted skill requiring an understanding of various definitions and a consideration of molecular structure and reaction context. While Arrhenius's definition offers a basic starting point, Brønsted-Lowry and particularly Lewis's definitions provide a more comprehensive and nuanced approach. By combining knowledge of these definitions and considering the overall structure and context, one can accurately predict the acidic or basic nature of a substance directly from its chemical formula. This skill is fundamental for anyone working in chemistry or related fields, offering crucial insights into reaction mechanisms and broader chemical systems. This understanding underpins a wide range of applications, from laboratory experiments to industrial processes and the study of life itself. Mastering this ability is crucial for developing a deeper appreciation and more thorough grasp of fundamental chemical principles.
Latest Posts
Latest Posts
-
Which Of The Following Are Ionic Compounds
Mar 30, 2025
-
Bacteria That Require Growth Factors And Complex Nutrients Are Termed
Mar 30, 2025
-
What Secretory Cell Type Is Found In The Adrenal Medulla
Mar 30, 2025
-
Types Of Repeat Signs In Music
Mar 30, 2025
-
What Is Considered The Basic Unit Of Life
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Identifying Acids And Bases By Their Chemical Formula . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
