Identifying The Important Intermolecular Forces In Pure Compounds
Muz Play
Mar 31, 2025 · 6 min read
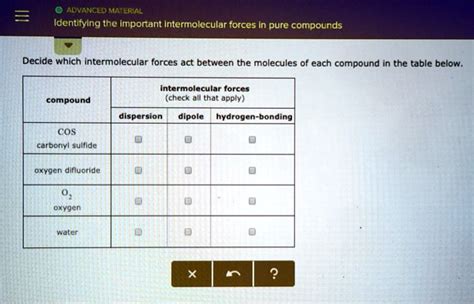
Table of Contents
Identifying the Important Intermolecular Forces in Pure Compounds
Understanding intermolecular forces (IMFs) is crucial for predicting the physical properties of pure compounds. These forces, weaker than the intramolecular bonds within molecules, govern properties like boiling point, melting point, viscosity, surface tension, and solubility. Accurately identifying the dominant IMFs in a pure compound allows for a deeper understanding of its behavior and interactions with other substances. This article provides a comprehensive guide to identifying the important intermolecular forces present in various pure compounds.
The Hierarchy of Intermolecular Forces
Intermolecular forces are broadly categorized in a hierarchy based on their strength:
1. Ion-Dipole Forces:
These are the strongest type of intermolecular force. They occur between an ion (either a cation or an anion) and a polar molecule. The ion's charge strongly attracts the oppositely charged end of the polar molecule. The strength of the interaction is directly proportional to the charge of the ion and the dipole moment of the molecule.
Example: NaCl dissolved in water. The Na⁺ ions are attracted to the partially negative oxygen atoms of water molecules, while the Cl⁻ ions are attracted to the partially positive hydrogen atoms.
2. Hydrogen Bonding:
A special type of dipole-dipole interaction, hydrogen bonds are exceptionally strong. They occur when a hydrogen atom bonded to a highly electronegative atom (typically fluorine, oxygen, or nitrogen) is attracted to another electronegative atom in a nearby molecule. The highly electronegative atom pulls electron density away from the hydrogen, creating a partially positive charge (δ⁺) on the hydrogen. This δ⁺ hydrogen then interacts strongly with the lone pairs of electrons on the electronegative atom of another molecule.
Example: Water (H₂O) molecules are extensively hydrogen-bonded. The partially positive hydrogen of one water molecule interacts with the partially negative oxygen of another. This strong hydrogen bonding accounts for water's high boiling point and other unique properties. Other examples include alcohols (R-OH), amines (R-NH₂), and carboxylic acids (R-COOH).
3. Dipole-Dipole Forces:
These forces arise between polar molecules. The partially positive end of one polar molecule is attracted to the partially negative end of another. The strength of the interaction is directly proportional to the dipole moments of the molecules involved.
Example: Acetone (CH₃COCH₃) molecules interact through dipole-dipole forces. The carbonyl group (C=O) creates a significant dipole moment, leading to relatively strong dipole-dipole interactions.
4. London Dispersion Forces (LDFs):
Also known as van der Waals forces, these are the weakest type of intermolecular force. They are present in all molecules, both polar and nonpolar. LDFs arise from temporary, instantaneous dipoles that occur due to the fluctuating electron distribution within a molecule. These temporary dipoles induce dipoles in neighboring molecules, leading to a weak attractive force. The strength of LDFs increases with the size and shape of the molecule. Larger molecules with more electrons have more easily polarizable electron clouds, resulting in stronger LDFs.
Example: Nonpolar molecules like methane (CH₄) only exhibit London dispersion forces. The strength of these forces is relatively weak, leading to a low boiling point for methane. However, larger nonpolar molecules like octane (C₈H₁₈) exhibit stronger LDFs due to their increased size and number of electrons.
Identifying IMFs in Pure Compounds: A Step-by-Step Approach
To identify the dominant intermolecular forces in a pure compound, follow these steps:
-
Determine the molecular structure: Draw the Lewis structure of the molecule to understand its geometry and bonding.
-
Identify the presence of polar bonds: Look for bonds between atoms with significantly different electronegativities. A significant difference in electronegativity leads to a polar bond.
-
Determine the molecular polarity: Based on the molecular geometry and the presence of polar bonds, determine whether the molecule is polar or nonpolar. A polar molecule has a net dipole moment, while a nonpolar molecule does not.
-
Identify the dominant intermolecular forces:
-
If the molecule is ionic: The dominant IMF is ion-dipole if it's dissolved in a polar solvent; otherwise, it's primarily ionic bonding within the solid lattice.
-
If the molecule contains O-H, N-H, or F-H bonds: Hydrogen bonding is the dominant IMF.
-
If the molecule is polar but does not contain O-H, N-H, or F-H bonds: Dipole-dipole forces are the dominant IMF.
-
If the molecule is nonpolar: London dispersion forces are the dominant IMF.
-
Examples: Identifying IMFs in Different Compounds
Let's analyze several examples to illustrate the process:
1. Water (H₂O):
- Molecular structure: Bent geometry.
- Polar bonds: O-H bonds are polar due to the large electronegativity difference between oxygen and hydrogen.
- Molecular polarity: Polar molecule due to the bent geometry and polar bonds.
- Dominant IMF: Hydrogen bonding (due to the presence of O-H bonds).
2. Methane (CH₄):
- Molecular structure: Tetrahedral geometry.
- Polar bonds: C-H bonds are relatively nonpolar.
- Molecular polarity: Nonpolar molecule due to the symmetrical tetrahedral geometry.
- Dominant IMF: London dispersion forces.
3. Ethanol (CH₃CH₂OH):
- Molecular structure: The hydroxyl group (-OH) is polar, while the ethyl group (-CH₂CH₃) is slightly polar due to the C-O bond.
- Polar bonds: O-H bond is highly polar; C-O bond exhibits some polarity.
- Molecular polarity: Polar molecule due to the presence of the polar -OH group.
- Dominant IMF: Hydrogen bonding (due to the presence of the O-H bond) although dipole-dipole interactions and LDFs also play a minor role.
4. Carbon Dioxide (CO₂):
- Molecular structure: Linear geometry.
- Polar bonds: C=O bonds are polar.
- Molecular polarity: Nonpolar molecule due to the symmetrical linear geometry (the dipole moments of the two C=O bonds cancel each other out).
- Dominant IMF: London dispersion forces.
5. Ammonia (NH₃):
- Molecular structure: Trigonal pyramidal geometry.
- Polar bonds: N-H bonds are polar.
- Molecular polarity: Polar molecule due to the pyramidal geometry and polar bonds.
- Dominant IMF: Hydrogen bonding (due to the presence of N-H bonds).
6. Bromine (Br₂):
- Molecular structure: Diatomic molecule.
- Polar bonds: Nonpolar bond (same atoms).
- Molecular polarity: Nonpolar molecule.
- Dominant IMF: London dispersion forces.
7. Acetic Acid (CH₃COOH):
- Molecular structure: Contains a carboxyl group (-COOH).
- Polar bonds: O-H and C=O bonds are polar.
- Molecular polarity: Polar molecule.
- Dominant IMF: Hydrogen bonding (strong interactions between the hydroxyl (-OH) groups of different molecules); dipole-dipole interactions and LDFs are also present.
The Influence of Molecular Size and Shape on IMFs
The strength of London dispersion forces significantly depends on the size and shape of the molecule. Larger molecules with more electrons have more polarizable electron clouds, resulting in stronger LDFs. Similarly, elongated molecules tend to exhibit stronger LDFs compared to compact molecules of similar molecular weight due to increased surface area contact. This explains why, for instance, long-chain alkanes have higher boiling points than their branched isomers. While all molecules experience LDFs, their relative importance increases as the size and complexity of the molecule increase, often becoming dominant even in the presence of weaker dipole-dipole interactions.
Conclusion
Identifying the important intermolecular forces in a pure compound is crucial for understanding its physical properties. By systematically analyzing the molecular structure and polarity, we can determine the dominant IMFs and predict various physical properties such as boiling point, melting point, and solubility. This knowledge is essential in various fields, including chemistry, materials science, and biochemistry. Remember that while this article focuses on identifying the dominant IMF, it is important to acknowledge the presence of other, weaker interactions which contribute to the overall intermolecular forces at play. A comprehensive understanding of these forces provides a powerful framework for predicting the behavior of molecules and materials.
Latest Posts
Latest Posts
-
Is The Five Carbon Sugar Found In Dna
Apr 01, 2025
-
Builds A New Dna Strand By Adding Complementary Bases
Apr 01, 2025
-
Which Of The Following Situations Will Lead To Natural Selection
Apr 01, 2025
-
How Does The Nucleus And Ribosomes Work Together
Apr 01, 2025
-
Having A Single Set Of Unpaired Chromosomes
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Identifying The Important Intermolecular Forces In Pure Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
