In Order To Break A Bond Energy Must Be
Muz Play
Mar 28, 2025 · 6 min read
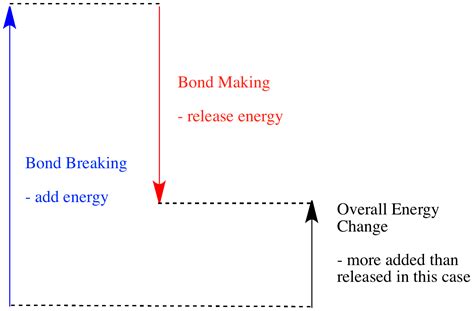
Table of Contents
In Order to Break a Bond, Energy Must Be… Supplied! Understanding Bond Energies and Chemical Reactions
Breaking chemical bonds is fundamental to countless processes, from digestion to the combustion of fuels. Understanding why energy input is necessary to break these bonds is key to comprehending chemical reactions and their energetics. This article delves deep into the concept of bond energy, exploring its definition, measurement, factors influencing it, and its crucial role in predicting reaction feasibility and spontaneity.
What is Bond Energy?
At its core, bond energy (also known as bond dissociation energy) represents the amount of energy required to break one mole of a specific type of bond in the gaseous phase. It's a measure of the strength of the bond – the higher the bond energy, the stronger the bond. This energy is typically expressed in kilojoules per mole (kJ/mol). It's important to note that we're talking about breaking the bond homolytically, meaning the electrons in the bond are equally divided between the two resulting fragments, creating radicals (atoms or groups with unpaired electrons).
Example: The Hydrogen-Hydrogen Bond
Consider the simplest molecule, hydrogen (H₂). The bond energy of the H-H bond is approximately 436 kJ/mol. This means that to break one mole of H-H bonds (that's 6.022 x 10²³ H-H bonds!), we need to supply 436 kJ of energy. This energy overcomes the attractive forces holding the two hydrogen atoms together.
Why Does Breaking a Bond Require Energy?
The necessity of energy input to break a bond stems from the fundamental nature of chemical bonding. Bonds are formed due to the attractive forces between atoms, resulting from electrostatic interactions. These attractive forces lower the overall energy of the system compared to the individual, isolated atoms. Therefore, to separate the atoms and break the bond, we need to add energy to overcome these attractive forces and return the system to a higher energy state.
The Potential Energy Curve
The relationship between the potential energy of two atoms and the distance between them is often depicted using a potential energy curve. This curve shows a minimum potential energy at a specific bond length, representing the most stable bond configuration. To break the bond, we must supply enough energy to raise the system's potential energy from this minimum to the point where the atoms are infinitely far apart (zero interaction).
Electrostatic Interactions: The Glue of Chemical Bonds
The attractive forces in chemical bonds arise from several types of electrostatic interactions:
- Ionic Bonds: These are primarily electrostatic attractions between oppositely charged ions (cations and anions). The energy required to break an ionic bond is dependent on the charges and sizes of the ions involved, following Coulomb's Law.
- Covalent Bonds: These involve the sharing of electrons between atoms. The electrons are attracted to the positive nuclei of both atoms, creating a region of high electron density that holds the atoms together. The strength of a covalent bond depends on factors like the electronegativity difference between the atoms and the number of electron pairs shared (single, double, or triple bonds).
- Metallic Bonds: In metals, valence electrons are delocalized and form a "sea" of electrons surrounding positively charged metal ions. The attractive forces between the positive ions and the electron sea hold the metal together.
Factors Affecting Bond Energy
Numerous factors influence the strength of a chemical bond and, consequently, its bond energy:
Bond Order
The number of electron pairs shared between two atoms (bond order) significantly impacts bond energy. For example, a triple bond (like in N₂) is significantly stronger than a double bond (like in O₂), which is stronger than a single bond (like in F₂). Higher bond order implies stronger attraction and higher bond energy.
Atomic Size
Larger atoms generally form weaker bonds because the valence electrons are further from the nucleus, resulting in weaker electrostatic attraction. This is why bond energies tend to decrease down a group in the periodic table.
Electronegativity
The electronegativity difference between bonded atoms also plays a crucial role. Bonds between atoms with similar electronegativities (nonpolar bonds) are generally stronger than bonds between atoms with significantly different electronegativities (polar bonds). However, this is not a universally applicable rule, and other factors can sometimes outweigh the impact of electronegativity.
Resonance
Molecules with resonance structures (delocalized electrons) often exhibit stronger bonds than those without resonance. The delocalization of electrons leads to a more stable and lower-energy system, increasing the energy required to break the bonds. Benzene is a classic example of a molecule with resonance structures that contribute to its high stability and relatively strong C-C bonds.
Hybridization
The hybridization of atomic orbitals involved in bond formation also influences bond energy. For instance, sp-hybridized orbitals form stronger bonds than sp²- or sp³-hybridized orbitals because of their greater s-character. This is related to the greater penetration of s orbitals closer to the nucleus and thus stronger attraction.
Bond Energy and Reaction Enthalpy
Bond energy plays a vital role in determining the enthalpy change (ΔH) of a chemical reaction. The enthalpy change is the difference in heat content between the products and reactants. It can be estimated using bond energies by considering the bonds broken in the reactants and the bonds formed in the products.
Hess's Law and Bond Energies
Hess's Law states that the enthalpy change of a reaction is independent of the pathway taken. Therefore, we can estimate the enthalpy change of a reaction by calculating the energy required to break all the bonds in the reactants (positive value, endothermic) and the energy released when forming the bonds in the products (negative value, exothermic). The overall enthalpy change is the sum of these energy changes.
Applications of Bond Energy
Understanding bond energies is crucial in various fields:
- Chemistry: Predicting reaction feasibility, determining activation energies, and studying reaction mechanisms.
- Materials Science: Designing and synthesizing new materials with desired properties based on bond strength and stability.
- Biochemistry: Understanding the energetics of biological processes such as enzyme catalysis and protein folding, which involve breaking and forming numerous chemical bonds.
- Chemical Engineering: Optimizing reaction conditions and designing efficient chemical processes based on energy considerations.
Conclusion: Energy Input – The Key to Bond Breaking
Breaking a chemical bond necessitates energy input. This fundamental principle is rooted in the attractive forces between atoms that form the chemical bond. The amount of energy needed, the bond energy, is influenced by a multitude of factors, including bond order, atomic size, electronegativity, resonance, and hybridization. By understanding bond energies and their impact on reaction energetics, we gain valuable insights into chemical reactions and their applications in various scientific disciplines. The detailed study of bond energies allows for a deeper comprehension of chemical reactions, paving the way for advancements in material science, biochemistry, and countless other applications where understanding molecular interactions is paramount. Furthermore, future research will likely refine our understanding of bond energy calculations and their predictive power, allowing for even more precise estimations of reaction energetics and enhancing our ability to manipulate chemical processes with greater efficiency and control.
Latest Posts
Latest Posts
-
What Is Held Constant In Gay Lussacs Law
Mar 31, 2025
-
Example Of A Line In A Poem
Mar 31, 2025
-
Interval Of Convergence Of A Taylor Series
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about In Order To Break A Bond Energy Must Be . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
