In Which Direction Will Water Molecules Move Across The Membrane
Muz Play
Mar 15, 2025 · 5 min read
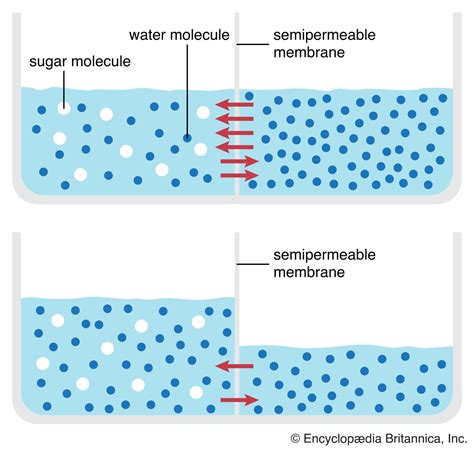
Table of Contents
In Which Direction Will Water Molecules Move Across a Membrane? Understanding Osmosis
The movement of water across a membrane is a fundamental process in biology, crucial for maintaining cellular function and overall organismal health. This movement, known as osmosis, is driven by the difference in water potential between two solutions separated by a selectively permeable membrane. Understanding the direction of water movement is key to grasping many biological phenomena, from plant turgor pressure to kidney function. This article will delve deep into the intricacies of osmosis, exploring the factors that influence water movement and the consequences of different osmotic conditions.
Understanding Water Potential
Before discussing the direction of water movement, it's essential to define water potential (Ψ). Water potential is the measure of the free energy of water, representing the tendency of water to move from one area to another. It's expressed in units of pressure (typically megapascals, MPa). Water potential is influenced by two primary factors:
1. Solute Potential (Ψ<sub>S</sub>)
This component reflects the effect of dissolved solutes on water potential. The presence of solutes lowers the water potential because solutes bind water molecules, reducing their availability for movement. A higher solute concentration results in a more negative solute potential. Pure water has a solute potential of zero.
2. Pressure Potential (Ψ<sub>P</sub>)
This component reflects the physical pressure on the water. Positive pressure potential (turgor pressure) pushes water out of a system, increasing its water potential. Negative pressure potential (tension) pulls water into a system, decreasing its water potential. For example, in plant cells, turgor pressure pushes the cell membrane against the cell wall, creating a positive pressure potential.
The total water potential (Ψ) is the sum of solute potential (Ψ<sub>S</sub>) and pressure potential (Ψ<sub>P</sub>):
Ψ = Ψ<sub>S</sub> + Ψ<sub>P</sub>
The Direction of Water Movement: The Osmosis Principle
Water always moves from an area of higher water potential to an area of lower water potential. This movement continues until the water potential is equal on both sides of the membrane. Several scenarios illustrate this principle:
1. Hypotonic Solution
A hypotonic solution has a higher water potential than the solution inside a cell. This means it has a lower solute concentration. When a cell is placed in a hypotonic solution, water moves into the cell, causing it to swell. If the cell wall is rigid, like in plant cells, this creates turgor pressure, preventing excessive swelling and lysis. However, animal cells lacking a cell wall may burst (lyse) in a hypotonic solution.
2. Hypertonic Solution
A hypertonic solution has a lower water potential than the solution inside a cell. This means it has a higher solute concentration. When a cell is placed in a hypertonic solution, water moves out of the cell, causing it to shrink (crenate in animal cells or plasmolyze in plant cells). This process can significantly impair cellular function.
3. Isotonic Solution
An isotonic solution has the same water potential as the solution inside a cell. There is no net movement of water across the membrane, and the cell maintains its size and shape. This is often an ideal situation for many cells.
Factors Affecting Water Movement Across Membranes
Several factors beyond water potential influence the rate and direction of water movement:
-
Membrane Permeability: The membrane's permeability to water determines how easily water molecules can cross it. Aquaporins, specialized protein channels, greatly enhance water permeability.
-
Temperature: Higher temperatures generally increase the rate of water movement due to increased kinetic energy of water molecules.
-
Surface Area: A larger surface area of the membrane allows for a faster rate of water movement.
-
Membrane Thickness: Thicker membranes offer greater resistance to water movement, slowing down the process.
Osmosis in Different Biological Systems
Osmosis plays a vital role in various biological systems:
1. Plant Cells: Turgor Pressure and Wilting
Plant cells rely on osmosis to maintain turgor pressure, the pressure exerted by the cell contents against the cell wall. This pressure provides structural support and keeps the plant upright. When plants are deprived of water, the cells lose water, turgor pressure decreases, and the plant wilts.
2. Animal Cells: Maintaining Cell Volume and Function
Animal cells maintain their volume and shape through osmotic balance. The kidneys play a crucial role in regulating the solute concentration of body fluids, ensuring that cells are bathed in an isotonic environment. Deviations from isotonicity can have severe consequences.
3. Water Absorption in Roots: The Role of Root Hair Cells
Root hair cells have a large surface area and are adapted to absorb water from the soil via osmosis. The soil solution typically has a higher water potential than the cells, driving water into the roots.
4. Kidney Function: Selective Reabsorption of Water
The kidneys regulate water balance in the body through selective reabsorption of water in the nephrons. The concentration gradient between the nephron tubules and the surrounding tissues drives water movement, ensuring proper hydration and waste excretion.
Osmosis and Reverse Osmosis: A Contrast
While osmosis describes the natural movement of water across a selectively permeable membrane, reverse osmosis is a process that uses external pressure to force water movement against its natural concentration gradient. This technology is used for water purification, removing dissolved salts and other impurities from water.
Applications of Osmosis Principles
Understanding osmosis has significant applications in various fields:
-
Agriculture: Irrigation techniques are designed to maintain optimal soil water potential for plant growth.
-
Medicine: Intravenous fluids are formulated to be isotonic to prevent damage to red blood cells. Dialysis treatments rely on osmosis to remove waste products from the blood.
-
Food Preservation: Osmosis is used in food preservation techniques like dehydration and pickling.
-
Environmental Science: Studying osmotic processes is crucial in understanding the behavior of aquatic organisms in different environments.
Conclusion: The Ubiquitous Nature of Osmosis
The direction of water movement across a membrane, governed by the principles of osmosis, is a fundamental process underpinning life itself. From the cellular level to the level of entire organisms, the ability of cells and tissues to regulate water movement is critical for their survival and function. Understanding the factors influencing this movement, including water potential and membrane properties, provides a framework for comprehending diverse biological phenomena and developing practical applications across various scientific and technological fields. Further research into the intricate details of osmosis continues to unlock new insights into the complexities of life and provides tools for developing innovative solutions in areas such as agriculture, medicine, and environmental science.
Latest Posts
Latest Posts
-
Evidence Of Light As A Particle
Mar 17, 2025
-
Do Achiral Molecules Have A Plane Of Symmetry
Mar 17, 2025
-
Acto 3 Escena 1 Romeo Y Julieta
Mar 17, 2025
-
Can You Be In Love With Two People
Mar 17, 2025
-
Factors That Influence The Elasticity Of Supply
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about In Which Direction Will Water Molecules Move Across The Membrane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
