Is A Solution A Homogeneous Mixture
Muz Play
Mar 16, 2025 · 5 min read
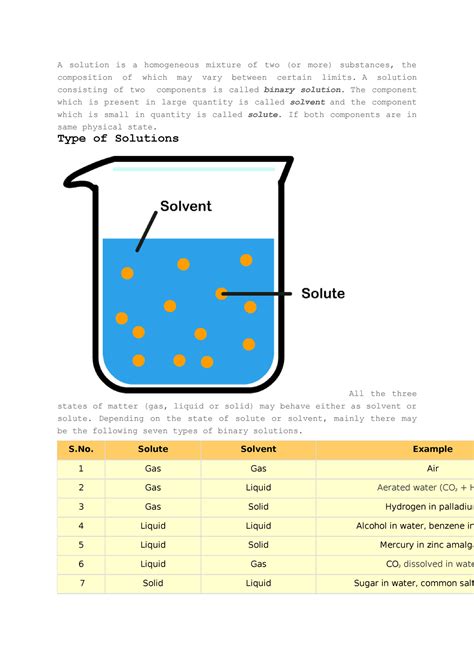
Table of Contents
Is a Solution a Homogeneous Mixture? A Deep Dive into Mixtures and Their Properties
The question of whether a solution is a homogeneous mixture is fundamentally important in chemistry and various scientific fields. The answer, simply put, is yes. However, understanding why requires a deeper exploration of the definitions of solutions, homogeneous mixtures, and the properties that distinguish them from other types of mixtures. This article will delve into these concepts, exploring the characteristics of solutions and homogeneous mixtures, providing examples, and clarifying common misconceptions.
Understanding Mixtures: A Foundation
Before diving into solutions, let's establish a firm understanding of mixtures. A mixture is a substance composed of two or more components not chemically bonded. This means the components retain their individual chemical properties and can be separated using physical methods like filtration, distillation, or evaporation. Crucially, the ratio of components in a mixture can vary. Mixtures are broadly categorized into two main types: homogeneous and heterogeneous.
Homogeneous Mixtures: Uniformity at the Molecular Level
A homogeneous mixture is characterized by its uniform composition throughout. At a macroscopic level (what we can see with the naked eye), it appears visually uniform. More importantly, at a microscopic level (down to the molecular level), the components are evenly distributed. This means no matter what sample you take from a homogeneous mixture, its composition will be identical. Examples include saltwater, air, and many alloys.
Heterogeneous Mixtures: A Lack of Uniformity
In contrast, a heterogeneous mixture displays non-uniform composition. Different parts of the mixture have visibly different properties or compositions. You can easily distinguish the individual components. Examples include sand and water, oil and water, and a salad.
Solutions: A Specific Type of Homogeneous Mixture
Now, let's focus on solutions. A solution is a specific type of homogeneous mixture where one substance, the solute, is dissolved in another substance, the solvent. The solute is typically present in a smaller amount than the solvent. The resulting mixture is a homogenous solution because the solute particles are dispersed uniformly throughout the solvent at a molecular level. This is unlike suspensions or colloids, where larger particles are dispersed, leading to non-uniformity.
Key Characteristics of Solutions:
-
Uniform Composition: As already mentioned, solutions exhibit uniform composition throughout. This uniformity extends to the microscopic level, with solute particles evenly distributed among the solvent molecules.
-
Particle Size: Solute particles in a solution are extremely small, typically at the atomic or molecular level. This ensures complete dissolution and a clear, transparent appearance (unless the solution is inherently colored due to the solute's properties).
-
Filtration: Solutions cannot be separated by simple filtration because the solute particles are too small to be trapped by filter paper. More advanced separation techniques, like distillation or chromatography, might be necessary.
-
Solubility: The ability of a solute to dissolve in a solvent is crucial. Solubility is influenced by factors like temperature, pressure (especially for gases), and the chemical nature of both solute and solvent – "like dissolves like" is a general rule (polar solvents dissolve polar solutes, nonpolar solvents dissolve nonpolar solutes).
-
Concentration: The amount of solute dissolved in a given amount of solvent or solution is defined as its concentration. Concentration can be expressed in various ways, such as molarity, molality, percentage by mass, or percentage by volume. These are important factors in various applications, from medicine to industrial processes.
Distinguishing Solutions from Other Homogeneous Mixtures
While all solutions are homogeneous mixtures, not all homogeneous mixtures are solutions. The key difference lies in the size and nature of the dispersed particles.
Comparison Table:
| Feature | Solution | Other Homogeneous Mixtures (e.g., alloys) |
|---|---|---|
| Particle Size | Atomic/Molecular | Can be larger (e.g., metallic atoms in alloys) |
| Separation | Requires advanced techniques (distillation, chromatography) | Might be possible with less sophisticated techniques |
| Appearance | Typically transparent | Can be opaque or translucent depending on the components |
| Solute-Solvent Interaction | Strong interactions; solute particles fully surrounded by solvent | Interactions can vary; not always a clear solute/solvent distinction |
For example, an alloy like brass (a mixture of copper and zinc) is a homogeneous mixture but not strictly a solution. While the components are uniformly distributed, the particles are larger than individual atoms or molecules. They are mixed at the metallic level, and their interactions are different from the solute-solvent interactions in a true solution.
Examples of Solutions in Everyday Life and Industry
Solutions are ubiquitous in our daily lives and essential in various industries. Here are a few examples:
-
Saltwater: Sodium chloride (salt) dissolved in water.
-
Sugar water: Sucrose (sugar) dissolved in water.
-
Air: A mixture of gases, primarily nitrogen and oxygen, where the gases are homogeneously mixed.
-
Brass: Although not a 'solution' in the strictest sense, it is a homogeneous mixture with components distributed uniformly.
-
Soda pop: Carbon dioxide gas dissolved in water with added sugar and flavorings.
-
Medical solutions: Many intravenous solutions are carefully prepared solutions containing electrolytes and other vital substances.
-
Pharmaceutical formulations: Many drugs are administered as solutions for efficient absorption.
-
Industrial processes: Solutions are integral to chemical reactions, plating processes, and many other industrial applications.
Misconceptions about Solutions
A common misconception is that solutions are always liquids. This is incorrect. While many common solutions are indeed liquids, solutions can also be in gaseous or solid states.
-
Gaseous solutions: Air is a classic example of a gaseous solution.
-
Solid solutions: Alloys like brass and stainless steel are examples of solid solutions. These are often formed by the uniform mixing of metal atoms at the atomic level.
Conclusion: Solutions as a Subset of Homogeneous Mixtures
In conclusion, the answer to the question, "Is a solution a homogeneous mixture?" is a resounding yes. Solutions represent a specific and important category within the broader classification of homogeneous mixtures. They are characterized by their uniform composition at the molecular level, small particle size of the solute, and complete dissolution of the solute in the solvent. Understanding the distinctions between solutions and other types of mixtures, along with their properties and applications, is critical in various scientific disciplines and daily life. The unique characteristics of solutions make them vital in diverse fields, from medicine and pharmaceuticals to industrial processes and environmental science.
Latest Posts
Latest Posts
-
Electric Potential From A Point Charge
Mar 17, 2025
-
Whats The Derivative Of A Constant
Mar 17, 2025
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
-
An Increase In The Aggregate Expenditures Schedule
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is A Solution A Homogeneous Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
