Is Alcohol An Acid Or Base
Muz Play
Mar 30, 2025 · 6 min read
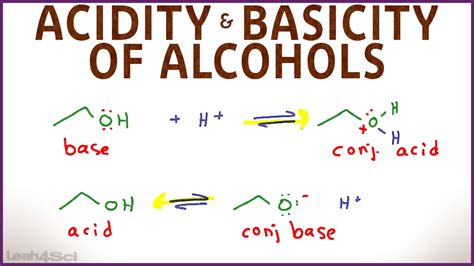
Table of Contents
Is Alcohol an Acid or a Base? Understanding the Chemistry of Alcohols
The question, "Is alcohol an acid or a base?" isn't as straightforward as it might seem. While the general answer leans towards weakly acidic, the reality is more nuanced and depends heavily on the specific alcohol in question and the context of the reaction. This in-depth exploration will delve into the chemical properties of alcohols, explaining their acidic and basic characteristics and the factors that influence their behavior.
The Amphoteric Nature of Alcohols
The crucial point to understand is that many alcohols are amphoteric. This means they can act as both acids and bases, depending on the circumstances. This dual nature stems from the structure of the alcohol molecule itself.
Alcohols as Weak Acids
The most common behavior of alcohols is their ability to act as weak acids. This acidity arises from the hydroxyl group (-OH) attached to the carbon atom. The oxygen atom in the hydroxyl group is highly electronegative, meaning it strongly attracts electrons. This pulls electron density away from the O-H bond, making the hydrogen atom slightly more positive (δ+) and therefore more readily released as a proton (H⁺).
When an alcohol acts as an acid, it donates a proton to a stronger base, forming an alkoxide ion. This reaction is typically shown as:
ROH + B⁻ ⇌ RO⁻ + HB
Where:
- ROH represents the alcohol
- B⁻ represents a strong base
- RO⁻ represents the alkoxide ion
- HB represents the conjugate acid of the base
The strength of this acidity varies considerably. For instance, methanol (CH₃OH) is a weaker acid than ethanol (CH₃CH₂OH), and both are considerably weaker than water. The relative acidity is influenced by several factors:
-
Inductive Effects: Electron-withdrawing groups attached to the carbon atom adjacent to the hydroxyl group increase the acidity. These groups pull electron density away from the O-H bond, making the proton more easily released. Conversely, electron-donating groups decrease acidity.
-
Steric Hindrance: Bulky groups around the hydroxyl group can hinder the approach of a base, reducing the rate of proton transfer and thus seemingly decreasing acidity.
-
Solvent Effects: The solvent in which the reaction takes place significantly impacts the acidity. Protic solvents (those containing O-H or N-H bonds) stabilize both the alcohol and the alkoxide ion, whereas aprotic solvents (lacking O-H or N-H bonds) tend to stabilize the alkoxide ion more effectively, increasing the apparent acidity.
Alcohols as Weak Bases
While less common, alcohols can also function as weak bases. In this case, the oxygen atom in the hydroxyl group accepts a proton from a strong acid. This reaction results in the formation of an oxonium ion.
ROH + H⁺ ⇌ ROH₂⁺
The basicity of alcohols is generally weaker than their acidity. The oxygen atom, although electronegative, possesses lone pairs of electrons that can accept a proton. However, this basicity is often overshadowed by their weak acidic properties. The lone pairs on the oxygen are less available for protonation compared to the readily available proton in the O-H bond for deprotonation.
Factors Influencing Alcohol Acidity/Basicity
The acidity and basicity of alcohols aren't fixed properties; they are highly dependent on the surrounding chemical environment.
The Role of the Alkyl Group
The nature of the alkyl group (R) attached to the hydroxyl group (-OH) significantly impacts the alcohol's acidity. As the alkyl group becomes larger and more branched, the acidity generally decreases. This is primarily due to the inductive effect and steric hindrance discussed earlier. Larger alkyl groups exert a stronger electron-donating effect, increasing electron density around the O-H bond and making proton release more difficult. Additionally, bulky alkyl groups hinder the approach of a base, slowing down the deprotonation reaction.
The Influence of Substituents
The presence of other substituents on the carbon chain also influences acidity. Electron-withdrawing groups, such as halogens (F, Cl, Br, I) or nitro groups (NO₂), increase acidity by stabilizing the negative charge on the alkoxide ion formed after proton donation. On the other hand, electron-donating groups decrease acidity.
Solvent Effects Revisited
The solvent plays a crucial role in determining the apparent acidity and basicity of alcohols. Protic solvents solvate both the alcohol and the alkoxide ion, while aprotic solvents preferentially solvate the alkoxide ion. This differential solvation affects the equilibrium of the acid-base reaction, making the alcohol appear more or less acidic depending on the solvent used.
Comparison with Other Compounds
To better understand the acidic and basic properties of alcohols, it's helpful to compare them to other classes of compounds.
Alcohols vs. Water
Water (H₂O) and alcohols share a similar structure, both possessing an -OH group. However, water is a more acidic than most alcohols. This is attributed to the smaller size of the hydrogen atom in water, which allows for better stabilization of the hydroxide ion (OH⁻) formed upon deprotonation. The absence of an alkyl group also contributes to water's higher acidity compared to alcohols.
Alcohols vs. Carboxylic Acids
Carboxylic acids (RCOOH) are significantly more acidic than alcohols. This difference stems from the presence of a carbonyl group (C=O) adjacent to the hydroxyl group in carboxylic acids. The carbonyl group strongly withdraws electrons from the O-H bond, making the proton significantly more acidic. The carboxylate ion (RCOO⁻) formed after deprotonation is also highly stabilized by resonance.
Alcohols vs. Amines
Amines (RNH₂, R₂NH, R₃N) are generally more basic than alcohols. The nitrogen atom in amines is less electronegative than the oxygen atom in alcohols, making it a better proton acceptor. The lone pair of electrons on the nitrogen atom is more readily available for protonation compared to the lone pairs on the oxygen atom in alcohols, resulting in stronger basicity.
Applications and Practical Implications
The acidic and basic properties of alcohols find numerous applications in various fields.
In Organic Synthesis
Alcohols are commonly used as reactants and solvents in organic synthesis. Their weak acidity allows them to react with strong bases to form alkoxides, which are powerful nucleophiles used in various reactions. Their weak basicity allows them to react with strong acids.
In Industrial Processes
Alcohols are used extensively in industrial processes as solvents, disinfectants, and raw materials for the production of other chemicals. Their acidity and basicity play a significant role in these processes, influencing reaction rates and product formation.
In Biological Systems
Alcohols play essential roles in biological systems. For instance, many sugars and carbohydrates contain hydroxyl groups, which contribute to their acidic properties and their ability to interact with other molecules. Furthermore, the alcohol group is fundamental to many biological molecules, impacting their function.
Conclusion: A Matter of Context
In conclusion, the question of whether alcohol is an acid or a base is not a simple yes or no answer. Alcohols are amphoteric, exhibiting both acidic and basic characteristics. Their behavior is highly context-dependent, influenced by the specific alcohol, the presence of other substituents, the solvent, and the reaction conditions. Understanding the factors affecting their acidity and basicity is crucial in various chemical applications and biological systems. While their acidity is generally more prominent than their basicity, the dual nature of alcohols adds to their versatility and importance in chemistry.
Latest Posts
Latest Posts
-
Protein Synthesis Takes Place In The
Apr 01, 2025
-
Microscopic Anatomy Of A Muscle Fiber
Apr 01, 2025
-
What Is The General Equation For Cellular Respiration
Apr 01, 2025
-
Are Influence Lines In The Fe Exam
Apr 01, 2025
-
The First Organisms That Oxygenated The Atmosphere Were
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Alcohol An Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
